Asia Pacific Surgical Equipment Market Summary
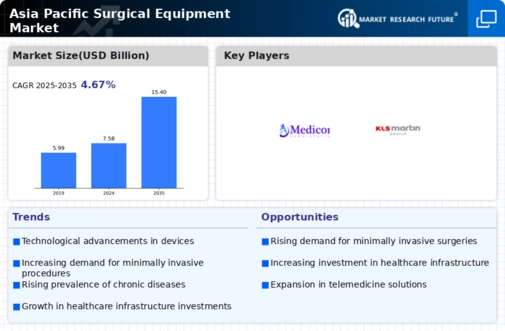
The Asia-Pacific Surgical Equipment market is projected to grow significantly from 7.58 USD billion in 2024 to 15.4 USD billion by 2035.
Key Market Trends & Highlights
Asia Pacific Surgical Equipment Market Key Trends and Highlights
- The market is expected to experience a compound annual growth rate of 6.64 percent from 2025 to 2035.
- By 2035, the market valuation is anticipated to reach 15.4 USD billion, indicating robust growth potential.
- In 2024, the market is valued at 7.58 USD billion, reflecting a strong foundation for future expansion.
- Growing adoption of advanced surgical technologies due to increasing healthcare expenditure is a major market driver.
Market Size & Forecast
| 2024 Market Size | 7.58 (USD Billion) |
| 2035 Market Size | 15.4 (USD Billion) |
| CAGR (2025-2035) | 6.64% |
Major Players
Integra LifeSciences Corporation, Boston Scientific Corporation, Smith & Nephew, B. Braun Melsungen AG, Ethicon LLC., Stryker Corporation, COVIDIEN, CONMED Corporation, MEDICON, Synergetics USA, Inc., Erbe Elektromedizin GmbH, KARL STORZ & Co., KLS Martin