Regulatory Support
Regulatory support plays a crucial role in the growth of the Artificial Pancreas Device System Market. Regulatory bodies are increasingly recognizing the importance of these devices in diabetes management, leading to streamlined approval processes. This support not only accelerates the introduction of innovative products but also instills confidence among manufacturers and consumers alike. Recent initiatives by regulatory agencies to establish clear guidelines for the development and testing of artificial pancreas systems suggest a commitment to fostering innovation in this field. As a result, the market is likely to see a surge in new entrants and product offerings, which could enhance competition and drive down costs for consumers.
Rising Diabetes Prevalence
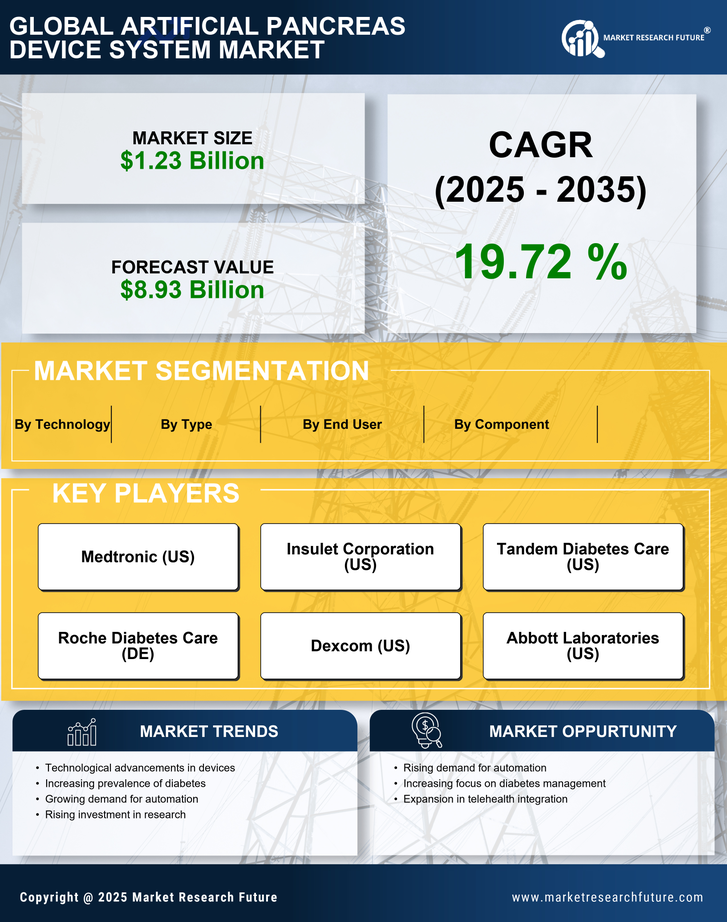
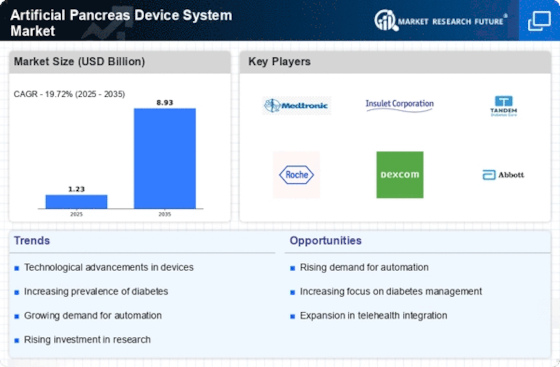
The rising prevalence of diabetes worldwide is a significant driver for the Artificial Pancreas Device System Market. With millions of individuals diagnosed with diabetes, the demand for effective management solutions is at an all-time high. Data indicates that the number of people living with diabetes is expected to reach 700 million by 2045, creating an urgent need for advanced treatment options. The artificial pancreas systems offer a promising solution by automating insulin delivery and monitoring blood glucose levels, thereby improving patient outcomes. This increasing patient population is likely to propel market growth, as healthcare providers seek to implement more efficient diabetes management strategies.
Technological Advancements
The Artificial Pancreas Device System Market is experiencing rapid technological advancements that enhance device functionality and user experience. Innovations such as continuous glucose monitoring and automated insulin delivery systems are becoming more sophisticated, allowing for better glycemic control. The integration of artificial intelligence and machine learning algorithms into these devices is likely to improve predictive capabilities, thus reducing the risk of hypoglycemia and hyperglycemia. According to recent data, the market for these advanced systems is projected to grow significantly, with estimates suggesting a compound annual growth rate of over 20% in the coming years. This trend indicates a strong demand for more efficient and user-friendly devices, which could potentially transform diabetes management for millions of patients.
Increased Awareness and Education
Increased awareness and education regarding diabetes management are pivotal in driving the Artificial Pancreas Device System Market. As healthcare professionals and patients become more informed about the benefits of advanced diabetes technologies, the adoption rates of artificial pancreas systems are expected to rise. Educational initiatives aimed at both patients and healthcare providers are likely to enhance understanding of how these devices can improve quality of life and reduce complications associated with diabetes. This heightened awareness may lead to greater acceptance and utilization of artificial pancreas systems, ultimately contributing to market expansion.
Investment in Research and Development
Investment in research and development is a key factor propelling the Artificial Pancreas Device System Market. As companies strive to innovate and improve existing technologies, substantial funding is being directed towards R&D efforts. This investment is likely to yield breakthroughs in device design, functionality, and user interface, making artificial pancreas systems more accessible and effective for patients. Furthermore, collaborations between academic institutions and industry players are fostering an environment conducive to innovation. The anticipated advancements resulting from these investments could significantly enhance the capabilities of artificial pancreas systems, thereby attracting more users and driving market growth.