North America : Innovation and Investment Hub
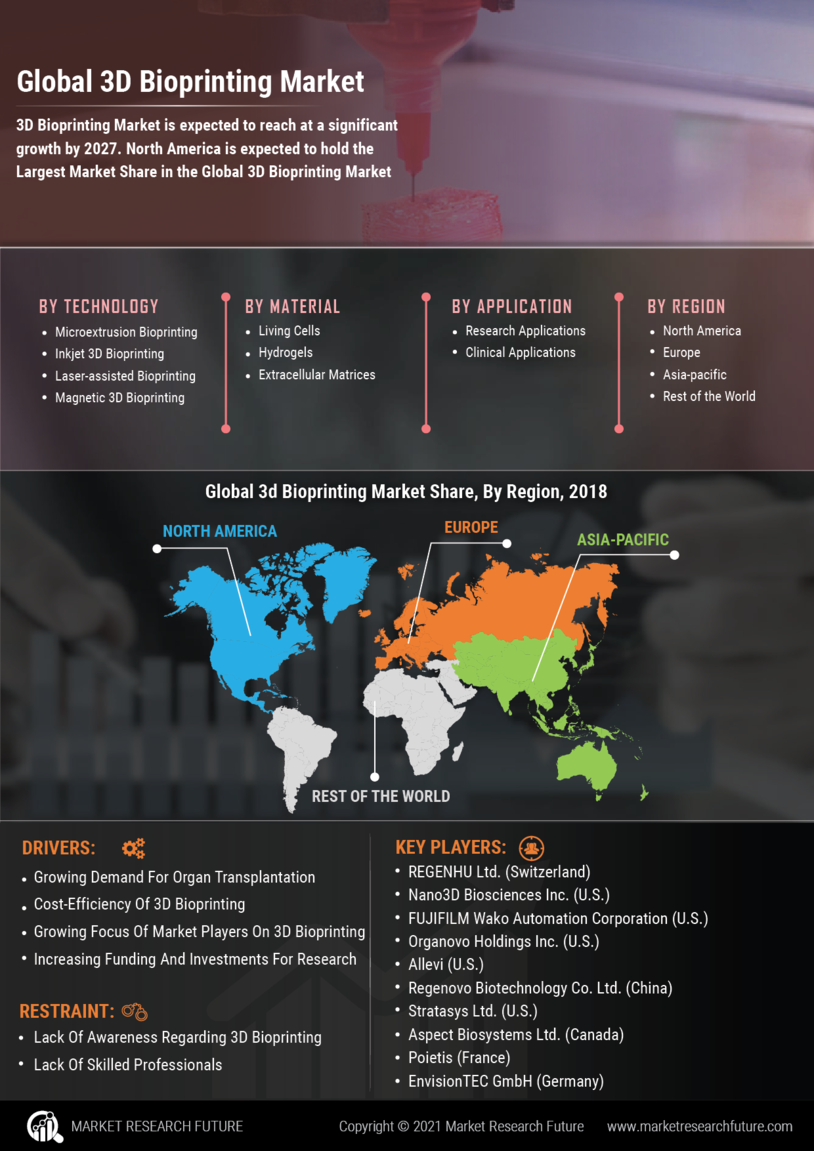
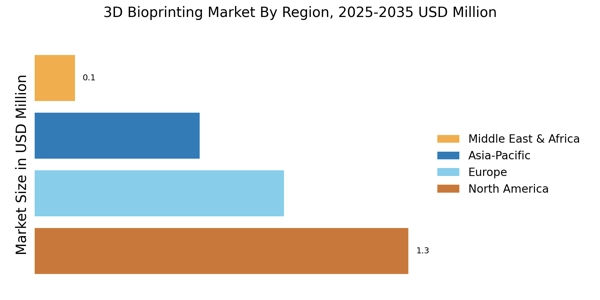
North America is the largest market for 3D bioprinting, holding approximately 45% of the global share. The region's growth is driven by significant investments in research and development, coupled with a robust healthcare infrastructure. Regulatory support from agencies like the FDA has catalyzed innovation, allowing for rapid advancements in bioprinting technologies. The increasing demand for organ transplants and personalized medicine further fuels market expansion.
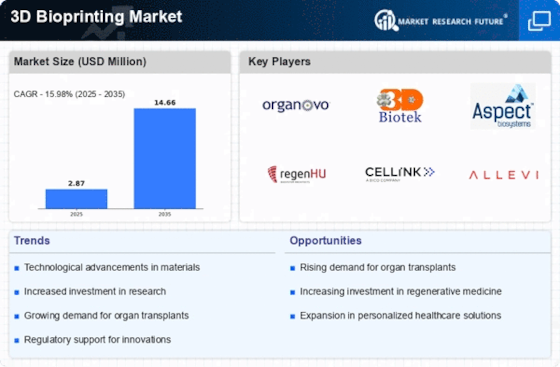
The United States is the dominant player, with key companies such as Organovo Holdings Inc and Allevi Inc leading the charge. Canada also plays a significant role, with firms like Aspect Biosystems Ltd contributing to the competitive landscape. The presence of established players and a growing number of startups enhances the region's innovation ecosystem, making it a focal point for advancements in bioprinting technology.
Europe : Emerging Regulatory Frameworks
Europe is the second-largest market for 3D bioprinting, accounting for around 30% of the global share. The region is witnessing growth driven by increasing investments in healthcare and biotechnology, alongside supportive regulatory frameworks.
The European Medicines Agency (EMA) is actively working on guidelines that promote the safe use of bioprinting technologies, which is expected to further stimulate market growth. The rising demand for regenerative medicine is also a key driver. Leading countries in Europe include Germany, the UK, and Switzerland, with Germany being the largest market.
Companies like CELLINK AB and EnvisionTEC GmbH are at the forefront, contributing to a competitive landscape characterized by innovation and collaboration. The presence of research institutions and universities enhances the region's capabilities, fostering partnerships that drive advancements in bioprinting applications.
Asia-Pacific : Rapid Growth and Adoption
Asia-Pacific is rapidly emerging as a significant player in the 3D bioprinting market, holding approximately 20% of the global share. The region's growth is fueled by increasing healthcare expenditures, a rising population, and advancements in technology. Countries like China and Japan are leading the charge, with government initiatives aimed at promoting bioprinting research and development. The demand for personalized medicine and organ transplants is also on the rise, driving market expansion.
China is the largest market in the region, with a growing number of startups and established companies entering the bioprinting space.
Japan follows closely, with significant investments in bioprinting technologies. The competitive landscape is characterized by a mix of local and international players, including Regenhu SA and 3D Biotek LLC, which are contributing to the region's innovation and growth in bioprinting applications.
Middle East and Africa : Emerging Market Dynamics
The Middle East and Africa region is gradually emerging in the 3D bioprinting market, currently holding about 5% of the global share. The growth is driven by increasing healthcare investments and a rising demand for advanced medical technologies. Countries like South Africa and the UAE are beginning to adopt bioprinting technologies, supported by government initiatives aimed at enhancing healthcare infrastructure.
The region's potential for growth is significant, particularly in regenerative medicine and tissue engineering. South Africa is leading the market in the region, with a growing number of research institutions focusing on bioprinting applications. The competitive landscape is still developing, with a few key players starting to emerge. The presence of international companies is also increasing, which is expected to drive innovation and collaboration in the region's bioprinting sector.