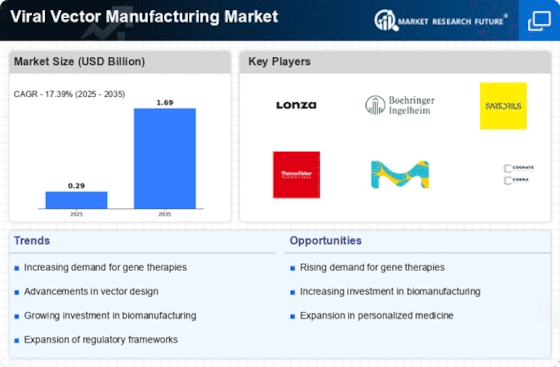
Market Share
Viral Vector Manufacturing Market Share Analysis
In this ever-changing world of viral vector manufacturing; companies adopt different strategies to be at the forefront of producing viral vectors for use in gene therapies, vaccine development as well other advanced medical applications. Fundamental strategy involves innovation in manufacturing technology. Companies spend heavily on R&D to scale up , improve quality ,and secure their methods for making these vectors.Cell culture systems, vector purification procedures or even vector delivery systems may be considered innovations. Cutting-edge solutions targeting developers involved in gene therapy and vaccine manufacturers are offered by such business leaders thus attracting customers searching for reliable high-quality therapeutic or preventive interventions with viral vectors.
Customization of the market share positioning in viral vector manufacturing depends on flexibility. There are various uses for gene therapies and vaccination, therefore companies design their production processes that can be adapted to different therapeutic groups, types of viruses and scales of production. For example, whether it is constructing gene editing vectors or oncolytic viruses or viral vector vaccines the use of customized solutions assists organizations in addressing issues encountered during manufacturing process.
Education and training serve as one of the tactics for market share positioning in Viral Vector Manufacturing Market. Thus companies incur costs associated with training bioprocess engineers, quality control teams and regulatory affairs personnel on different aspects related to viral vector production technologies thereby ensuring that users are competent enough while following protocols stated by standard operating procedures (SOPs). They teach technology implementation methods along with process optimization techniques among others. In fact these firms equip users with knowledge about how to manufacture viral vectors hence this move results into improvement in brand loyalty so that there is increase in their respective market shares.
Scalability and cost-effectiveness are key tactics for market share positioning in the Viral Vector Manufacturing Market. As gene therapies become commercialized while vaccine production goes up, companies are focusing on providing solutions that meet large scale production needs at low costs. Single-use technology, closed system operation as well as fast downstream purification are the main scalable and cost-effective viral vector manufacturing concepts. For this reason, scalability and cost effectives have become the most attractive factors of commercial application since they expand the customer base and disseminate viral vector technology. Specifically, by overcoming difficulties associated with translating research into large-scale commercial viral vectors manufacturing such an approach enables penetration of the market share.
















Leave a Comment