Focus on Quality Control
Quality control remains a critical aspect in the production of pharmaceuticals and biopharmaceuticals, influencing the prepacked chromatography-columns market. The need for stringent quality assurance measures has led to an increased adoption of prepacked columns, which provide consistent performance and reproducibility. In 2025, the market for quality control in laboratories is expected to grow by 15%, further emphasizing the importance of reliable chromatography solutions. As regulatory bodies enforce stricter guidelines, the demand for prepacked chromatography-columns is likely to increase, as they facilitate compliance with these standards while ensuring product integrity.
Emergence of Personalized Medicine
The rise of personalized medicine is reshaping the landscape of the prepacked chromatography-columns market. As healthcare shifts towards tailored treatments, the need for efficient separation and purification techniques becomes paramount. Prepacked columns offer the flexibility and precision required for the development of personalized therapies. In 2025, the personalized medicine market is anticipated to exceed $100 billion, which may significantly impact the demand for chromatography solutions. This trend suggests that the prepacked chromatography-columns market will benefit from the growing focus on individualized treatment options, as researchers and manufacturers seek to optimize their processes.
Rising Biopharmaceutical Production
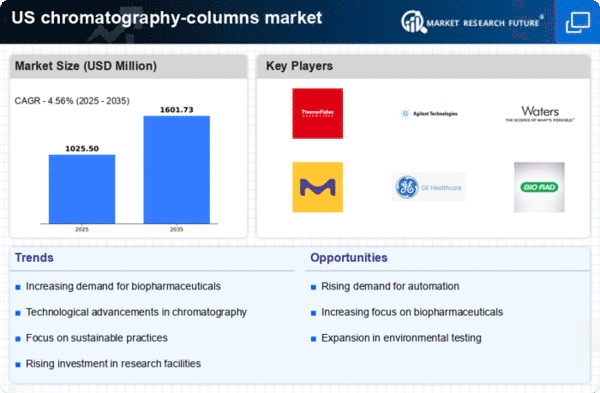
growth is due to the increasing production of biopharmaceuticals in the US. As biopharmaceuticals require highly efficient purification processes, the demand for prepacked columns is likely to rise. In 2025, the biopharmaceutical sector is projected to reach a market value of approximately $300 billion, which could drive the need for advanced chromatography solutions. The efficiency and reproducibility offered by prepacked columns make them a preferred choice for manufacturers. This trend indicates a robust future for the prepacked chromatography-columns market, as companies seek to streamline their production processes and ensure compliance with regulatory standards.
Regulatory Compliance and Standardization
Regulatory compliance and standardization are increasingly influencing the prepacked chromatography-columns market. As regulatory agencies impose stricter guidelines on pharmaceutical manufacturing, the need for reliable and consistent chromatography solutions becomes essential. Prepacked columns are designed to meet these regulatory requirements, ensuring that manufacturers can maintain compliance while optimizing their processes. In 2025, the market for regulatory compliance solutions in the pharmaceutical industry is expected to grow by 12%, highlighting the importance of prepacked chromatography-columns in meeting these challenges. This trend indicates a sustained demand for prepacked solutions as companies strive to adhere to evolving regulations.
Increased Investment in Research and Development
Investment in research and development (R&D) is a driving force behind the growth of the prepacked chromatography-columns market. As pharmaceutical companies and research institutions allocate more resources to R&D, the demand for advanced chromatography solutions is likely to rise. In 2025, R&D spending in the pharmaceutical sector is projected to reach $200 billion, indicating a strong commitment to innovation. This trend suggests that prepacked chromatography-columns will play a crucial role in facilitating new discoveries and improving existing processes, thereby enhancing the overall efficiency of drug development.