Growing Patient Advocacy Groups
The Dravet syndrome market is influenced by the emergence of robust patient advocacy groups. These groups play a pivotal role in raising awareness and driving research initiatives. These organizations are instrumental in educating the public and healthcare professionals about the challenges faced by individuals with dravet syndrome. By fostering community engagement and collaboration, patient advocacy groups can influence policy decisions and funding allocations for research. Their efforts may lead to increased visibility for the dravet syndrome market, encouraging pharmaceutical companies to invest in the development of new therapies. As these groups continue to advocate for patients' needs, the overall landscape of the dravet syndrome market is likely to evolve, resulting in improved treatment options and support for affected families.
Advancements in Genetic Research
The Dravet syndrome market is poised for expansion. This expansion is due to advancements in genetic research that continue to unveil the underlying mechanisms of the disorder. With the identification of specific genetic mutations associated with dravet syndrome, targeted therapies are being developed that may offer improved efficacy and safety profiles. This scientific progress not only enhances treatment options but also fosters collaboration between pharmaceutical companies and research institutions. As a result, the dravet syndrome market is likely to witness an influx of novel therapies aimed at addressing the unique needs of patients. Moreover, the potential for personalized medicine approaches could further stimulate market growth, as treatments become increasingly tailored to individual genetic profiles.
Rising Prevalence of Dravet Syndrome
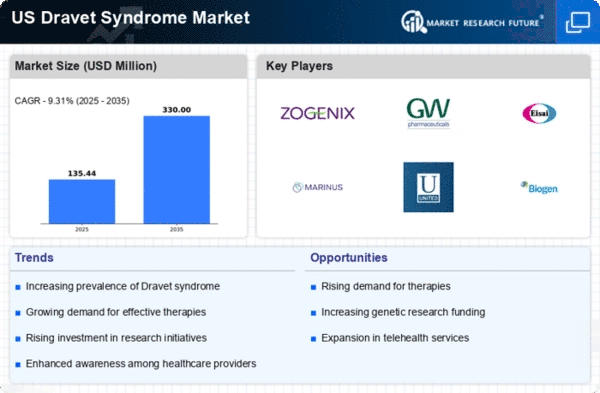
The Dravet syndrome market is experiencing growth. This growth is due to the increasing prevalence of this rare genetic epilepsy disorder in the US. Recent estimates suggest that dravet syndrome affects approximately 1 in 15,700 live births, leading to a significant patient population requiring specialized treatment. As awareness of the condition rises among healthcare professionals and families, the demand for effective therapies is likely to increase. This growing patient base is expected to drive investments in research and development, ultimately expanding the dravet syndrome market. Furthermore, the increasing recognition of the long-term impact of dravet syndrome on patients' quality of life may prompt healthcare systems to allocate more resources towards innovative treatment options, thereby enhancing market dynamics.
Regulatory Support for Innovative Treatments
The Dravet syndrome market benefits from a favorable regulatory environment. This environment encourages the development of innovative treatments. Regulatory agencies in the US, such as the FDA, have established pathways for expedited approval of therapies targeting rare diseases, including dravet syndrome. This regulatory support is crucial for pharmaceutical companies seeking to bring new therapies to market quickly. The designation of orphan drug status for certain treatments can also provide financial incentives, such as tax credits and market exclusivity, which may enhance the attractiveness of investing in the dravet syndrome market. As more companies pursue the development of novel therapies, the overall market landscape is likely to evolve, offering patients access to a broader range of treatment options.
Increased Investment in Rare Disease Research
The Dravet syndrome market is experiencing a surge in investment. This surge occurs as stakeholders recognize the unmet medical needs associated with rare diseases. Venture capital firms and pharmaceutical companies are increasingly directing funds towards research and development initiatives focused on dravet syndrome. This influx of capital is expected to accelerate the pace of innovation, leading to the discovery of new therapies and treatment modalities. Additionally, public-private partnerships may emerge, further enhancing research efforts in the dravet syndrome market. As funding increases, the potential for breakthroughs in treatment options becomes more pronounced, ultimately benefiting patients and healthcare providers alike.