Government Initiatives and Funding
Government initiatives aimed at improving healthcare access and funding for rare diseases significantly impact the dravet syndrome market. In France, the government has allocated substantial resources to support research and treatment for rare conditions, including Dravet syndrome. This funding is crucial for developing new therapies and enhancing patient care. The French National Health Authority has implemented policies to streamline the approval process for innovative treatments, which may lead to quicker access for patients. As a result, the dravet syndrome market benefits from increased investment and collaboration among stakeholders, fostering an environment conducive to advancements in treatment options.
Rising Incidence of Dravet Syndrome
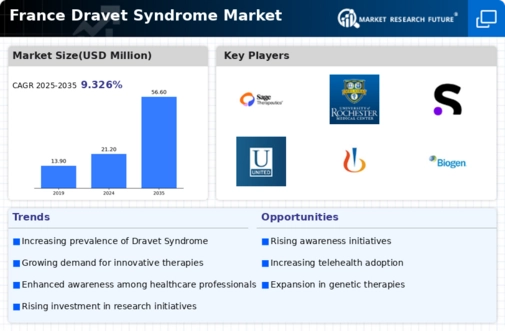
The increasing incidence of Dravet syndrome in France is a notable driver for the dravet syndrome market. Recent studies indicate that the prevalence of this rare genetic epilepsy disorder is approximately 1 in 15,700 live births. This rising incidence necessitates enhanced healthcare services and treatment options, thereby expanding the market. As more cases are diagnosed, the demand for specialized therapies and support systems grows. This trend is likely to stimulate investment in research and development, as pharmaceutical companies seek to address the unmet needs of patients. Consequently, the dravet syndrome market is poised for growth, with stakeholders focusing on innovative solutions to manage this complex condition.
Increased Research and Clinical Trials
The surge in research and clinical trials focused on Dravet syndrome is a critical driver for the dravet syndrome market. In France, numerous clinical studies are underway, exploring novel therapeutic approaches and potential drug candidates. This heightened research activity is fueled by the need for effective treatments, as existing options may not adequately address the complexities of the condition. The involvement of academic institutions and pharmaceutical companies in these trials fosters collaboration and accelerates the development of new therapies. As a result, the dravet syndrome market is likely to experience growth, driven by the continuous influx of innovative treatment options.
Growing Patient Advocacy and Support Groups
The emergence of patient advocacy and support groups in France plays a pivotal role in shaping the dravet syndrome market. These organizations raise awareness about the condition, provide resources for families, and advocate for better treatment options. Their efforts contribute to increased visibility of Dravet syndrome, which may lead to more diagnoses and a greater demand for effective therapies. Furthermore, these groups often collaborate with healthcare professionals and researchers, facilitating the exchange of information and promoting clinical trials. This dynamic interaction enhances the overall landscape of the dravet syndrome market, driving innovation and improving patient outcomes.
Technological Advancements in Treatment Delivery
Technological advancements in treatment delivery systems are transforming the dravet syndrome market. Innovations such as wearable devices and telemedicine platforms enable more effective monitoring and management of patients with Dravet syndrome. These technologies facilitate real-time data collection, allowing healthcare providers to tailor treatments to individual needs. In France, the integration of digital health solutions is gaining traction, potentially improving adherence to treatment regimens. As these technologies become more prevalent, they may enhance the overall efficacy of therapies, thereby expanding the dravet syndrome market. The potential for improved patient outcomes through technology is a compelling driver for market growth.