Supportive Regulatory Framework
A supportive regulatory framework is emerging in the UK, which is conducive to the growth of the nanomedicine market. Regulatory bodies are increasingly recognizing the unique challenges and opportunities presented by nanomedicine, leading to the establishment of guidelines that facilitate the approval process for new products. This evolving landscape is expected to enhance the speed at which innovative therapies reach the market. In 2025, the UK government is anticipated to implement new policies aimed at streamlining the regulatory pathway for nanomedicine products, thereby encouraging investment and fostering innovation. As a result, the nanomedicine market is likely to benefit from a more efficient regulatory environment, promoting the development of cutting-edge therapies.
Growing Demand for Targeted Therapies
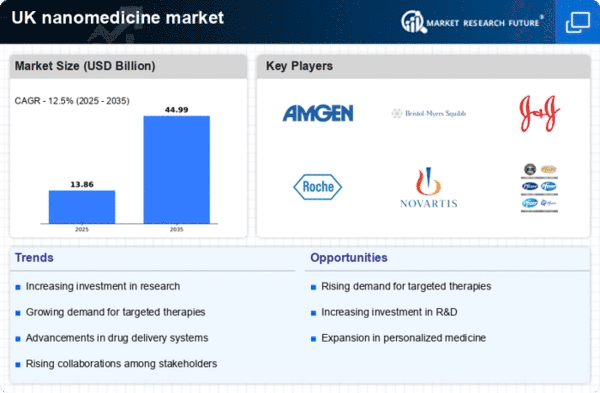
The demand for targeted therapies is on the rise within the UK, significantly impacting the nanomedicine market. Patients and healthcare providers are increasingly seeking treatment options that minimize side effects while maximizing therapeutic efficacy. Nanomedicine offers the potential for highly targeted drug delivery systems that can specifically address disease sites, thereby improving treatment outcomes. In 2025, it is estimated that targeted therapies will account for over 40% of the total oncology market in the UK, highlighting a shift towards precision medicine. This trend is likely to drive further investment and innovation in the nanomedicine sector, as companies strive to develop more effective and personalized treatment options.
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases in the UK is a significant driver for the nanomedicine market. Conditions such as cancer, diabetes, and cardiovascular diseases are becoming more prevalent, necessitating the development of advanced treatment modalities. According to recent statistics, chronic diseases account for nearly 70% of all deaths in the UK, underscoring the urgent need for effective therapeutic solutions. Nanomedicine offers promising avenues for targeted drug delivery and improved therapeutic efficacy, which could potentially transform patient outcomes. As healthcare providers seek innovative approaches to manage these diseases, the demand for nanomedicine solutions is expected to grow, thereby propelling the market forward.
Technological Advancements in Nanotechnology
Technological advancements in nanotechnology are playing a pivotal role in shaping the nanomedicine market. Innovations in nanomaterials and nanocarriers are enhancing the efficacy and safety of drug delivery systems. For instance, the development of nanoparticles that can cross biological barriers is revolutionizing the way medications are administered. In 2025, the market for nanotechnology in healthcare is anticipated to exceed £2 billion in the UK, driven by these advancements. This growth is indicative of the increasing recognition of nanotechnology's potential to improve therapeutic outcomes and reduce side effects. As research continues to unveil new applications, the nanomedicine market is likely to expand significantly.
Rising Investment in Research and Development
The nanomedicine market in the UK is experiencing a surge in investment, particularly in research and development (R&D). This trend is driven by both public and private sectors, with funding allocations increasing to support innovative projects. In 2025, R&D expenditure in the life sciences sector is projected to reach approximately £5 billion, reflecting a growing commitment to advancing nanomedicine technologies. This influx of capital is likely to facilitate the development of novel therapeutic agents and diagnostic tools, thereby enhancing the overall landscape of the nanomedicine market. Furthermore, collaborations between universities and industry players are becoming more prevalent, fostering an environment conducive to innovation and technological breakthroughs.