Supportive Regulatory Framework
Germany's supportive regulatory framework is playing a pivotal role in the growth of the nanomedicine market. The regulatory bodies are actively working to streamline the approval processes for nanomedicine products, ensuring that they meet safety and efficacy standards while facilitating timely market entry. This proactive approach is encouraging innovation and investment in the sector, as companies feel more confident in navigating the regulatory landscape. Furthermore, the establishment of clear guidelines for the development and commercialization of nanomedicine products is fostering a conducive environment for research and collaboration. As a result, the nanomedicine market is expected to benefit from an influx of new products and technologies, enhancing its overall competitiveness.
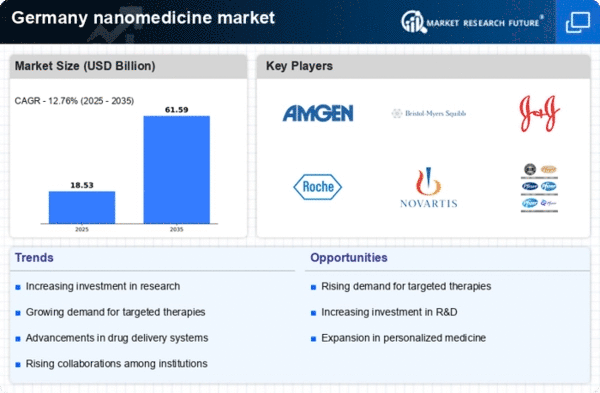
Rising Demand for Targeted Therapies
The increasing prevalence of chronic diseases in Germany is driving the demand for targeted therapies, which are a hallmark of the nanomedicine market. As healthcare providers seek more effective treatment options, the ability of nanomedicine to deliver drugs directly to affected cells is becoming increasingly appealing. Reports indicate that the market for targeted therapies is expected to grow at a CAGR of approximately 10% over the next five years. This growth is likely to be fueled by advancements in nanotechnology, which enhance the efficacy and safety of treatments. Consequently, the rising demand for these therapies is propelling the expansion of the nanomedicine market in Germany, as stakeholders aim to meet the needs of an aging population and improve patient outcomes.
Aging Population and Healthcare Needs
The demographic shift in Germany, characterized by an aging population, is creating a pressing need for innovative healthcare solutions, particularly in the nanomedicine market. As the elderly population grows, the incidence of age-related diseases such as cancer, cardiovascular disorders, and neurodegenerative conditions is expected to rise. This trend necessitates the development of advanced therapeutic options that can address complex health issues effectively. The nanomedicine market is poised to respond to these challenges by offering targeted and personalized treatment modalities. It is estimated that the market could expand by over 15% in the next few years, driven by the urgent healthcare needs of this demographic.
Investment in Research and Development
Germany's commitment to innovation is evident in its substantial investment in research and development (R&D) within the nanomedicine market. The government and private sector are channeling significant funds into R&D initiatives, with expenditures reaching approximately €3 billion annually. This investment is aimed at fostering breakthroughs in drug formulation, delivery systems, and diagnostic tools. As a result, the landscape of nanomedicine is evolving rapidly, with new products and technologies emerging that promise to enhance treatment efficacy. The focus on R&D not only supports the growth of the nanomedicine market but also positions Germany as a leader in the field, attracting international collaborations and partnerships.
Technological Advancements in Nanotechnology
Technological advancements in nanotechnology are significantly influencing the nanomedicine market in Germany. Innovations in nanomaterials, such as nanoparticles and nanocarriers, are enhancing drug delivery systems, making them more efficient and effective. These advancements are not only improving the bioavailability of drugs but also reducing side effects, which is crucial for patient compliance. The integration of artificial intelligence and machine learning in the development of nanomedicine is also gaining traction, potentially revolutionizing the way treatments are designed and administered. As these technologies continue to evolve, they are likely to propel the growth of the nanomedicine market, with projections indicating a potential market size increase of €1 billion by 2027.