Growing Focus on Patient Safety
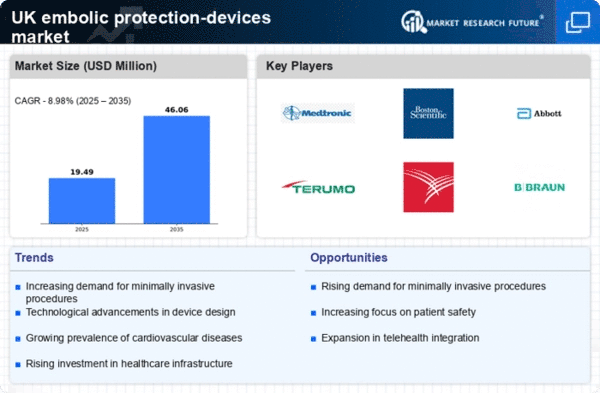
The heightened emphasis on patient safety within the UK healthcare system is a significant driver for the embolic protection-devices market. As healthcare providers strive to minimize complications during invasive procedures, the use of embolic protection devices has become a standard practice. This focus on safety is driven by both regulatory bodies and patient advocacy groups, which advocate for the implementation of best practices in medical procedures. The increasing awareness of the potential risks associated with embolic debris has led to a greater acceptance of these devices among clinicians. Consequently, the market is expected to experience robust growth, as more healthcare facilities prioritize the use of embolic protection devices to enhance patient outcomes and reduce the incidence of adverse events.
Supportive Reimbursement Policies
The presence of supportive reimbursement policies in the UK is a crucial factor driving the embolic protection-devices market. The National Health Service (NHS) has been increasingly recognizing the importance of embolic protection during high-risk procedures, leading to the inclusion of these devices in reimbursement schedules. This financial support encourages healthcare providers to adopt embolic protection devices, as it alleviates the cost burden associated with their use. As a result, hospitals and clinics are more likely to invest in these technologies, knowing that they will be reimbursed for their expenses. The positive impact of reimbursement policies is evident, as the market is projected to grow by approximately 6% over the next few years, reflecting the increasing integration of embolic protection devices into standard clinical practice.
Technological Innovations in Device Design
Innovations in the design and functionality of embolic protection devices are significantly influencing the embolic protection-devices market. Manufacturers are increasingly focusing on developing advanced devices that offer improved efficacy and safety profiles. For instance, the introduction of next-generation filters and balloon occlusion devices has enhanced the ability to capture and remove embolic debris during procedures. These advancements not only improve patient safety but also increase procedural success rates. The UK market has witnessed a surge in the adoption of these innovative devices, with a projected growth rate of around 8% annually over the next five years. As healthcare professionals become more aware of the benefits of these technologies, the demand for sophisticated embolic protection devices is likely to rise, further propelling market growth.
Rising Incidence of Cardiovascular Diseases
The increasing prevalence of cardiovascular diseases in the UK is a primary driver for the embolic protection-devices market. As the population ages, the incidence of conditions such as atrial fibrillation and coronary artery disease rises, leading to a higher demand for procedures that require embolic protection. According to recent statistics, cardiovascular diseases account for approximately 27% of all deaths in the UK, underscoring the urgent need for effective treatment options. This growing patient population necessitates the use of embolic protection devices during interventions like transcatheter aortic valve replacement (TAVR) and percutaneous coronary interventions (PCI). Consequently, the embolic protection-devices market is expected to expand as healthcare providers seek to mitigate the risks associated with these procedures, ensuring better patient outcomes and reducing the likelihood of complications.
Increasing Investment in Healthcare Infrastructure
The ongoing investment in healthcare infrastructure in the UK is positively impacting the embolic protection-devices market. As the government and private sector allocate more resources to enhance medical facilities and technologies, the availability of advanced embolic protection devices is likely to improve. This investment is particularly evident in the expansion of cardiac care units and interventional cardiology departments, which are crucial for the effective use of these devices. With an estimated £20 billion earmarked for healthcare improvements over the next five years, the embolic protection-devices market stands to benefit from enhanced access to cutting-edge technologies. As healthcare providers upgrade their facilities and equipment, the adoption of embolic protection devices is expected to rise, contributing to overall market growth.