Supportive Reimbursement Policies
The presence of supportive reimbursement policies in Germany is a significant driver for the embolic protection-devices market. The German healthcare system has established frameworks that facilitate the coverage of innovative medical devices, including embolic protection technologies. This financial backing encourages hospitals and clinics to adopt these devices, as they can be assured of reimbursement for their use in relevant procedures. Consequently, the embolic protection-devices market is likely to experience growth, as healthcare providers are more inclined to invest in advanced technologies that improve patient outcomes while being financially viable.
Rising Demand for Enhanced Patient Safety
The increasing emphasis on patient safety in medical procedures is a critical driver for the embolic protection-devices market. As healthcare standards evolve, there is a growing expectation for devices that minimize risks during interventions such as cardiac surgeries. The focus on reducing complications and improving recovery times has led to a surge in the adoption of embolic protection devices. In Germany, healthcare facilities are prioritizing technologies that enhance patient safety, which is likely to result in a robust growth trajectory for the embolic protection-devices market. This trend reflects a broader commitment to quality care and patient-centered approaches in the healthcare system.
Technological Innovations in Device Design
Innovations in the design and functionality of embolic protection devices are significantly influencing the market in Germany. Recent advancements have led to the development of more efficient and user-friendly devices, enhancing their effectiveness during surgical procedures. For instance, the introduction of next-generation filters and balloons has improved the capture of embolic debris, thereby reducing complications. The embolic protection-devices market is projected to grow as these technological enhancements not only improve patient outcomes but also increase the adoption rates among healthcare professionals. Furthermore, the integration of digital technologies, such as real-time imaging and monitoring, is likely to further drive market expansion.
Increasing Incidence of Cardiovascular Diseases
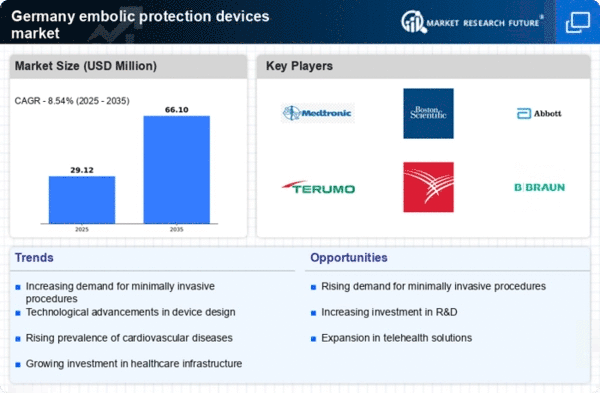
The rising prevalence of cardiovascular diseases in Germany is a primary driver for the embolic protection-devices market. As the population ages, the incidence of conditions such as atrial fibrillation and coronary artery disease has escalated. Reports indicate that approximately 30% of the German population over 65 years is affected by cardiovascular issues, necessitating advanced medical interventions. This trend is likely to propel the demand for embolic protection devices, which are crucial during procedures like transcatheter aortic valve replacement (TAVR). The embolic protection-devices market is expected to witness substantial growth as healthcare providers seek to mitigate the risks associated with these procedures, ensuring patient safety and improving outcomes.
Growing Awareness and Education Among Healthcare Professionals
There is a notable increase in awareness and education regarding the benefits of embolic protection devices among healthcare professionals in Germany. Medical institutions are investing in training programs and workshops to familiarize practitioners with the latest advancements in embolic protection technology. This educational push is crucial, as it equips healthcare providers with the knowledge to effectively utilize these devices during high-risk procedures. As a result, the embolic protection-devices market is expected to expand, driven by a more informed medical community that recognizes the importance of these devices in enhancing patient safety and procedural success.