Evolving Regulatory Frameworks
Regulatory influences play a crucial role in shaping the Tramadol Drug Market. Governments and health authorities are continuously updating guidelines and regulations surrounding the prescription of opioids and analgesics. In many regions, Tramadol is classified as a controlled substance, which necessitates careful monitoring and prescription practices. However, the evolving regulatory landscape may also lead to increased acceptance of Tramadol as a safer alternative to traditional opioids. This shift could potentially expand its market share, as healthcare providers seek to comply with regulations while effectively managing patient pain. The balance between regulation and accessibility is pivotal in determining the future trajectory of the Tramadol Drug Market.
Shifts in Patient Demographics
Shifts in patient demographics, particularly the aging population, are influencing the Tramadol Drug Market. As the global population ages, the prevalence of age-related conditions that require pain management is expected to rise. Older adults often experience chronic pain due to various health issues, leading to an increased demand for effective analgesics like Tramadol. Additionally, younger demographics are also experiencing higher rates of pain-related conditions, which may further expand the market. This demographic shift suggests that the Tramadol Drug Market must adapt to meet the evolving needs of diverse patient populations, potentially leading to tailored marketing strategies and product offerings.
Advancements in Pharmaceutical Research
Innovation in pharmaceutical research is a significant driver of the Tramadol Drug Market. Ongoing studies into the efficacy and safety of Tramadol formulations are likely to yield new insights that enhance its therapeutic profile. For instance, research into extended-release formulations may improve patient compliance and pain management outcomes. Additionally, the exploration of combination therapies that include Tramadol could provide more comprehensive pain relief options. As the pharmaceutical industry invests in research and development, the potential for novel Tramadol products to enter the market may stimulate growth and diversify offerings within the Tramadol Drug Market.
Rising Awareness of Pain Management Options
The increasing awareness of pain management options among patients and healthcare providers is a notable driver of the Tramadol Drug Market. Educational initiatives and campaigns aimed at informing the public about chronic pain and its treatment options have gained traction. This heightened awareness encourages patients to seek medical advice and explore various analgesic options, including Tramadol. As a result, healthcare professionals are more likely to prescribe Tramadol for pain management, contributing to its market growth. Furthermore, the emphasis on patient-centered care and shared decision-making in treatment plans may further enhance the adoption of Tramadol in clinical practice, thereby positively impacting the Tramadol Drug Market.
Increasing Prevalence of Chronic Pain Conditions
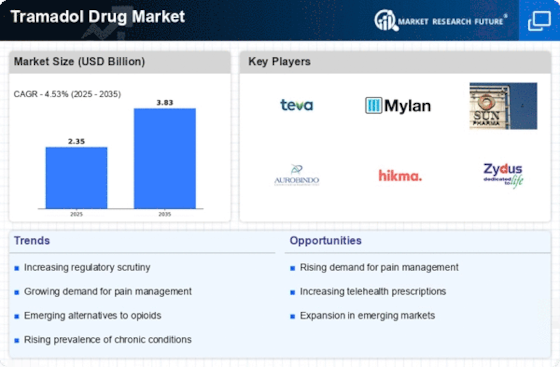
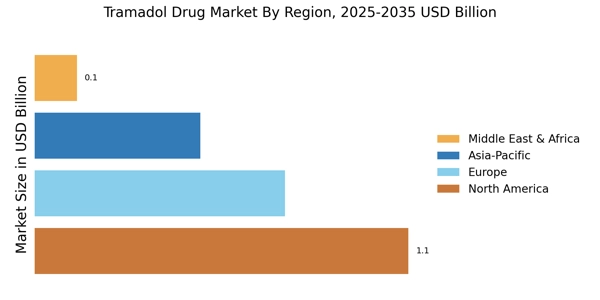
The rising incidence of chronic pain conditions, such as arthritis and fibromyalgia, is a primary driver of the Tramadol Drug Market. As populations age and lifestyle-related ailments become more common, the demand for effective pain management solutions intensifies. According to recent estimates, chronic pain affects approximately 20% of adults, leading to a substantial market for analgesics like Tramadol. This trend suggests that healthcare providers are increasingly turning to Tramadol as a viable option for managing moderate to severe pain, thereby bolstering its market presence. Furthermore, the growing awareness of pain management strategies among patients and healthcare professionals is likely to enhance the adoption of Tramadol, contributing to the overall growth of the Tramadol Drug Market.