Rising Incidence of Cancer
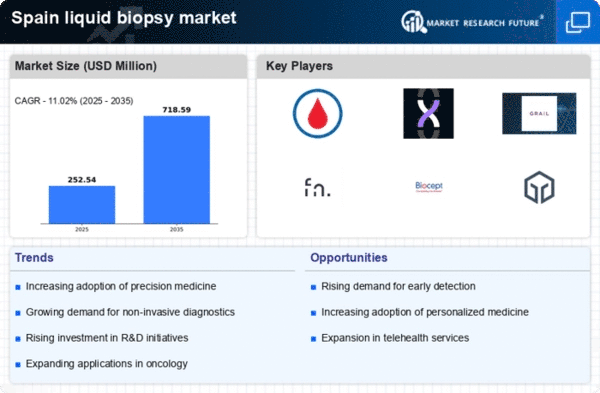
The increasing incidence of cancer in Spain is a primary driver for the liquid biopsy market. According to recent statistics, cancer cases are projected to rise by approximately 20% over the next decade. This alarming trend necessitates innovative diagnostic methods, such as liquid biopsies, which offer non-invasive alternatives to traditional tissue biopsies. The liquid biopsy market is likely to benefit from this growing demand, as healthcare providers seek efficient and effective ways to detect and monitor cancer. Furthermore, the ability of liquid biopsies to provide real-time insights into tumor dynamics may enhance patient management and treatment outcomes, thereby solidifying their role in oncology practices across Spain.
Regulatory Framework Enhancements
The regulatory landscape for liquid biopsies in Spain is evolving, with authorities actively working to streamline approval processes for new diagnostic tests. This supportive regulatory environment is crucial for the liquid biopsy market, as it facilitates quicker access to innovative technologies for healthcare providers. Recent initiatives by the Spanish Agency of Medicines and Medical Devices aim to establish clear guidelines for the validation and commercialization of liquid biopsy products. As a result, the market is likely to experience accelerated growth, with more companies entering the space and contributing to the development of novel diagnostic solutions that meet regulatory standards.
Advancements in Diagnostic Technologies
Technological innovations in diagnostic tools are propelling the liquid biopsy market forward. The integration of next-generation sequencing (NGS) and advanced bioinformatics has significantly improved the sensitivity and specificity of liquid biopsies. In Spain, the market is expected to witness a compound annual growth rate (CAGR) of around 15% over the next five years, driven by these advancements. The liquid biopsy market is increasingly adopting these technologies to enhance early detection and personalized treatment strategies. As healthcare systems prioritize precision medicine, the demand for liquid biopsies is likely to surge, reflecting a shift towards more tailored therapeutic approaches in oncology.
Increased Focus on Personalized Medicine
The shift towards personalized medicine is significantly influencing the liquid biopsy market in Spain. Healthcare professionals are increasingly recognizing the value of tailored treatment plans based on individual patient profiles. Liquid biopsies provide critical information regarding tumor genetics and dynamics, enabling oncologists to make informed decisions about therapy options. This trend is expected to drive the liquid biopsy market, as more clinicians adopt these tests to enhance patient outcomes. With a growing emphasis on precision oncology, the demand for liquid biopsies is likely to expand, reflecting a broader transformation in cancer care that prioritizes individualized treatment strategies.
Growing Investment in Research and Development
Investment in research and development (R&D) within the liquid biopsy market is on the rise in Spain. Public and private sectors are increasingly funding initiatives aimed at enhancing liquid biopsy technologies and expanding their applications. This trend is indicative of a broader commitment to improving cancer diagnostics and treatment. The liquid biopsy market is expected to see substantial growth as new products emerge from these R&D efforts. With an estimated €200 million allocated to cancer research in Spain, the potential for innovative liquid biopsy solutions is significant. This influx of funding may lead to breakthroughs that could redefine cancer management and monitoring.