Rising Healthcare Expenditure
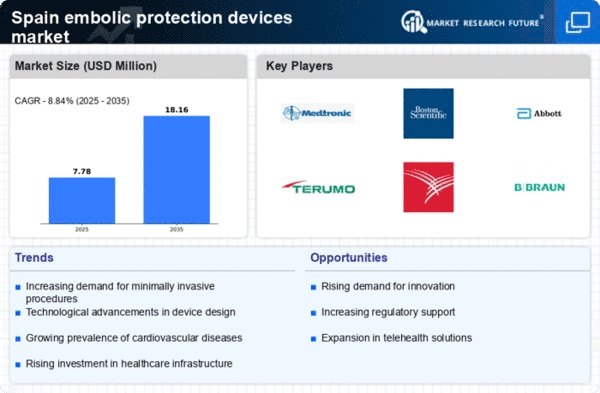
Upward trend in healthcare expenditure in Spain is poised to positively impact the embolic protection-devices market. With healthcare spending projected to reach approximately 10% of GDP, there is a growing emphasis on investing in advanced medical technologies. This increase in funding allows healthcare facilities to procure high-quality embolic protection devices, which are essential for improving patient safety during cardiovascular procedures. Moreover, as hospitals strive to meet international standards of care, the adoption of these devices is likely to become more prevalent. The focus on enhancing healthcare quality and patient outcomes may drive further growth in the embolic protection-devices market.
Government Initiatives and Funding
Government initiatives aimed at improving healthcare infrastructure in Spain are likely to drive the embolic protection-devices market. The Spanish government has allocated substantial funding to enhance cardiovascular care, which includes the procurement of advanced medical devices. This financial support is crucial for hospitals and clinics, enabling them to invest in state-of-the-art embolic protection devices. Additionally, public health campaigns focused on cardiovascular health are expected to raise awareness among healthcare providers and patients alike, further stimulating demand. The commitment to improving patient outcomes through better technology may lead to a more robust market environment for embolic protection devices in the coming years.
Rising Incidence of Cardiovascular Diseases
The increasing prevalence of cardiovascular diseases in Spain is a primary driver for the embolic protection-devices market. According to recent health statistics, cardiovascular diseases account for approximately 30% of all deaths in the country. This alarming trend necessitates advanced medical interventions, including embolic protection devices, to mitigate risks during procedures such as carotid artery stenting. The demand for these devices is expected to rise as healthcare providers seek to enhance patient outcomes and reduce complications associated with cardiovascular interventions. Furthermore, the Spanish healthcare system is increasingly prioritizing preventive measures, which may further bolster the adoption of embolic protection devices in clinical settings.
Technological Innovations in Medical Devices
Technological advancements in medical devices are significantly influencing the embolic protection-devices market. Innovations such as improved filter designs and biocompatible materials enhance the efficacy and safety of these devices. In Spain, the market has witnessed a surge in the introduction of next-generation embolic protection devices that offer better performance and ease of use. For instance, devices that incorporate real-time imaging capabilities allow for more precise placement and monitoring during procedures. This trend is likely to attract more healthcare professionals to adopt these advanced solutions, thereby expanding the market. The integration of digital technologies in device functionality may also lead to increased investment in research and development within the industry.
Aging Population and Increased Surgical Procedures
Spain's aging population is a significant factor contributing to the growth of the embolic protection-devices market. As the demographic shifts towards an older age group, the incidence of conditions requiring surgical interventions, such as carotid artery disease, is likely to increase. Older patients often present with multiple comorbidities, necessitating the use of embolic protection devices during surgical procedures to minimize the risk of embolic events. The Spanish healthcare system is adapting to this demographic change by enhancing surgical capabilities and investing in advanced medical technologies. This trend suggests a sustained demand for embolic protection devices as the number of surgical procedures rises in the coming years.