Focus on Data Security and Compliance
Data security and compliance are critical concerns for organizations operating in the etmf systems market. In South America, the rise in cyber threats and data breaches has prompted companies to prioritize the protection of sensitive information. Regulatory bodies are enforcing stricter guidelines, necessitating the implementation of robust etmf systems that ensure data integrity and security. In 2025, it is anticipated that investments in data security solutions will increase by 15%, reflecting the urgency to safeguard clinical trial data. Organizations are seeking systems that not only comply with local regulations but also offer advanced security features. This focus on data protection is likely to drive the adoption of etmf systems, as companies strive to mitigate risks associated with data management and maintain compliance with evolving regulatory frameworks.
Emergence of Local Players and Startups
The emergence of local players and startups in the etmf systems market is reshaping the competitive landscape in South America. These companies are introducing innovative solutions tailored to the specific needs of the region, fostering a more dynamic market environment. In 2025, it is estimated that local startups will capture approximately 20% of the market share, driven by their agility and ability to adapt to local regulations. This influx of new entrants is encouraging established players to enhance their offerings and invest in research and development. The presence of local players not only stimulates competition but also promotes the development of customized solutions that address unique challenges faced by organizations in South America. As a result, the growth of local startups is likely to be a significant driver in the etmf systems market.
Increased Investment in Clinical Trials
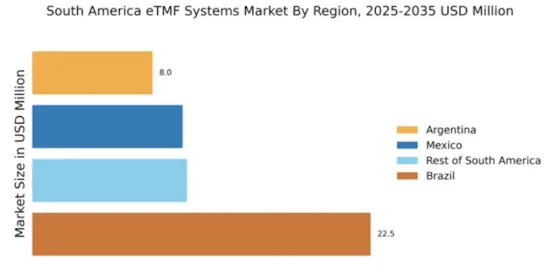
The surge in clinical trial activities across South America significantly influences the etmf systems market. With a growing number of pharmaceutical companies and research organizations establishing operations in the region, the demand for efficient trial management solutions is escalating. In 2025, the clinical trial market in South America is expected to reach approximately $1.5 billion, driving the need for advanced etmf systems. These systems facilitate better tracking, management, and reporting of trial data, which is crucial for meeting regulatory standards. Furthermore, the competitive landscape compels organizations to adopt innovative technologies that enhance trial efficiency. As a result, the integration of etmf systems is becoming increasingly vital for organizations aiming to optimize their clinical trial processes and ensure compliance with local regulations.
Growing Adoption of Cloud-Based Solutions
The shift towards cloud-based solutions is transforming the etmf systems market in South America. Organizations are increasingly recognizing the benefits of cloud technology, including scalability, cost-effectiveness, and enhanced collaboration. By 2025, it is projected that the cloud computing market in the region will exceed $10 billion, indicating a strong trend towards digital transformation. Cloud-based etmf systems offer real-time access to data, facilitating better decision-making and collaboration among stakeholders. This trend is particularly relevant for organizations involved in multi-site clinical trials, where seamless data sharing is essential. As companies seek to improve operational efficiency and reduce costs, the adoption of cloud-based etmf systems is likely to accelerate, positioning them as a key driver in the market.
Rising Demand for Efficient Data Management
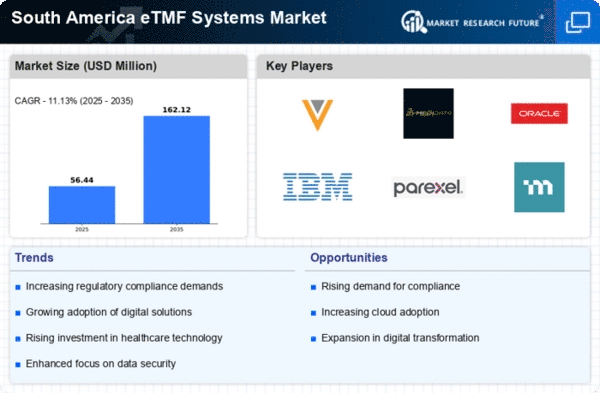
The increasing need for efficient data management solutions is a primary driver in the etmf systems market. Organizations in South America are recognizing the importance of streamlined data handling to enhance operational efficiency. As regulatory requirements become more stringent, the demand for electronic trial master files is expected to rise. In 2025, the market is projected to grow at a CAGR of 12%, reflecting a shift towards digital solutions. Companies are investing in etmf systems to ensure compliance and improve data accessibility. This trend is particularly evident in the pharmaceutical and clinical research sectors, where the need for accurate and timely data is paramount. The emphasis on data integrity and security further propels the adoption of these systems, indicating a robust growth trajectory for the etmf systems market in the region.