Rising Demand for Clinical Trials
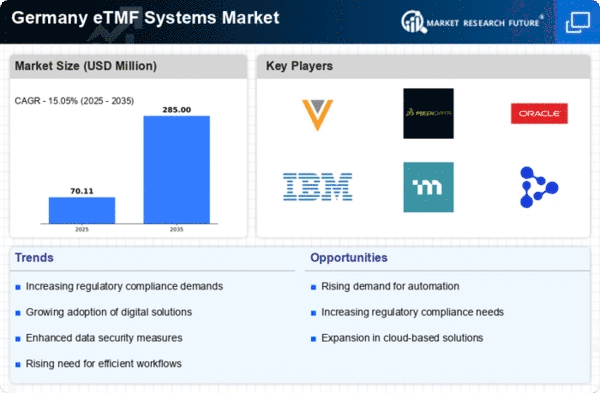
The etmf systems market in Germany is experiencing a notable surge in demand for clinical trials. This increase is driven by the growing need for innovative therapies and the expansion of research activities across various therapeutic areas. As pharmaceutical and biotechnology companies seek to streamline their clinical trial processes, the adoption of electronic trial master file (eTMF) systems becomes essential. In 2025, the market is projected to grow at a CAGR of approximately 15%, reflecting the urgency for efficient data management and regulatory compliance. The etmf systems market is thus positioned to benefit from this trend, as organizations prioritize the need for real-time access to trial data and improved collaboration among stakeholders.
Emphasis on Operational Efficiency
Operational efficiency remains a critical driver for the etmf systems market in Germany. Organizations are increasingly recognizing the importance of optimizing their processes to reduce costs and enhance productivity. By implementing eTMF systems, companies can automate document management, streamline workflows, and minimize the risk of errors. This shift towards digital solutions is expected to lead to a reduction in operational costs by up to 20% over the next few years. The etmf systems market is likely to see a rise in adoption as organizations strive to achieve greater efficiency and effectiveness in their clinical operations, ultimately improving their competitive edge.
Growing Focus on Patient-Centric Approaches
The etmf systems market in Germany is witnessing a growing focus on patient-centric approaches in clinical research. As stakeholders increasingly prioritize patient engagement and experience, the demand for systems that facilitate better communication and data sharing is on the rise. eTMF systems play a pivotal role in ensuring that patient data is accurately captured and easily accessible, thereby enhancing the overall research process. This trend is expected to drive market growth, with projections indicating an increase in investment in patient-centric technologies by approximately 30% in the coming years. The etmf systems market is thus adapting to meet these evolving needs, ensuring that patient perspectives are integrated into clinical trial designs.
Technological Advancements in Data Management
Technological advancements are significantly influencing the etmf systems market in Germany. The integration of artificial intelligence (AI) and machine learning (ML) into eTMF systems is enhancing data management capabilities, allowing for more efficient data analysis and reporting. These innovations are expected to improve the accuracy and speed of data processing, which is crucial for regulatory submissions. As organizations increasingly adopt these advanced technologies, the etmf systems market is likely to experience substantial growth, with estimates suggesting a market expansion of around 25% by 2026. This trend underscores the importance of staying at the forefront of technological developments to maintain competitiveness.
Increased Investment in Research and Development
Investment in research and development (R&D) is a key driver for the etmf systems market in Germany. With the government and private sector allocating substantial funds towards R&D initiatives, there is a heightened demand for efficient data management solutions. The etmf systems market is poised to benefit from this trend, as organizations seek to enhance their capabilities in managing clinical trial documentation and compliance. In 2025, R&D spending in the pharmaceutical sector is projected to reach €10 billion, further fueling the need for robust eTMF systems. This investment not only supports innovation but also ensures that companies can meet regulatory requirements effectively.