Emphasis on Cost Reduction Strategies
Cost reduction remains a pivotal driver in the etmf systems market. Organizations are increasingly seeking solutions that not only streamline operations but also minimize expenses associated with clinical trials. The adoption of etmf systems is seen as a strategic move to achieve these objectives, as they facilitate better resource allocation and reduce the need for extensive manual processes. The market is anticipated to grow at a rate of around 11% as companies recognize the financial benefits of implementing these systems.. By leveraging etmf solutions, organizations can enhance their operational efficiency, ultimately leading to lower trial costs and improved profitability.
Rising Focus on Patient-Centric Approaches
The eTMF systems market is experiencing a significant shift towards patient-centric approaches in clinical trials.. This trend reflects a broader movement within the healthcare sector to prioritize patient engagement and experience. As organizations strive to enhance patient recruitment and retention, the demand for etmf systems that facilitate real-time data collection and patient feedback is increasing. The market is expected to see a growth rate of around 10% annually as stakeholders recognize the value of integrating patient perspectives into trial designs.. By adopting etmf systems that support these initiatives, companies can improve trial efficiency and outcomes, ultimately leading to more successful product launches and better healthcare solutions.
Technological Advancements in Clinical Trials
Technological advancements are significantly influencing the etmf systems market in the UK. Innovations such as artificial intelligence and machine learning are being integrated into etmf systems, enhancing data analysis and decision-making processes. These technologies enable organizations to process large datasets more efficiently, thereby reducing the time and costs associated with clinical trials. The market is projected to grow by approximately 15% over the next few years, driven by the increasing adoption of these advanced technologies.. As companies seek to optimize their clinical trial processes, the demand for sophisticated etmf systems that incorporate these innovations is likely to rise, positioning the market for substantial growth.
Increased Demand for Data Management Solutions
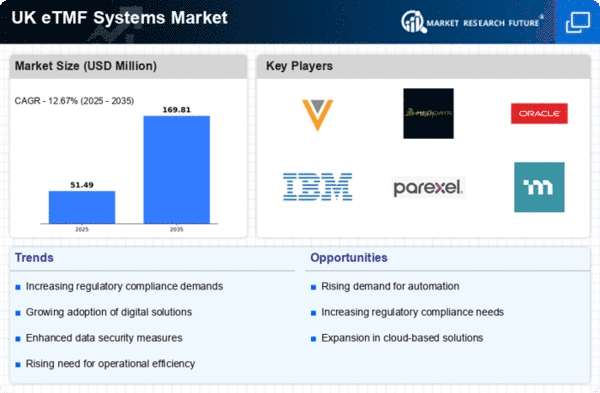
The etmf systems market in the UK is experiencing heightened demand for robust data management solutions. This trend is driven by the need for efficient handling of vast amounts of clinical trial data. As the pharmaceutical and biotechnology sectors expand, the requirement for streamlined data management systems becomes critical. The market is projected to grow at a CAGR of approximately 12% over the next five years, indicating a strong shift towards digital solutions.. Companies are increasingly investing in etmf systems to enhance data integrity and accessibility, which are essential for regulatory compliance and operational efficiency. This growing emphasis on data management is likely to propel the etmf systems market forward, as organizations seek to leverage technology for better decision-making and improved patient outcomes.
Growing Regulatory Scrutiny and Compliance Needs
The eTMF systems market is impacted by growing regulatory scrutiny within the UK healthcare sector.. As regulatory bodies impose stricter guidelines on clinical trial processes, organizations are compelled to adopt etmf systems that ensure compliance with these regulations. This trend is likely to drive market growth, with an expected increase of about 9% in the coming years. Companies are investing in etmf solutions that provide comprehensive audit trails and data security features, which are essential for meeting regulatory requirements. The heightened focus on compliance not only enhances the credibility of clinical trials but also fosters trust among stakeholders, further propelling the etmf systems market.