Regulatory Support for Orphan Drugs
Regulatory support for orphan drugs is emerging as a significant driver for the Pulmonary Alveolar Proteinosis Drug Market. Many countries have established incentives to encourage the development of treatments for rare diseases, including PAP. These incentives often include tax breaks, extended market exclusivity, and expedited review processes. Such supportive regulatory frameworks are likely to attract pharmaceutical companies to invest in the development of therapies for PAP. As a result, the market may witness an influx of innovative drugs designed to address the unique challenges posed by this condition. This regulatory environment not only fosters competition but also enhances the likelihood of successful drug approvals, ultimately benefiting patients in need of effective treatments within the Pulmonary Alveolar Proteinosis Drug Market.
Advancements in Diagnostic Techniques
Advancements in diagnostic techniques for Pulmonary Alveolar Proteinosis are significantly influencing the Pulmonary Alveolar Proteinosis Drug Market. Enhanced imaging modalities and improved laboratory tests have led to earlier and more accurate diagnoses of PAP. This progress not only facilitates timely treatment but also raises awareness about the condition among healthcare providers. As diagnostic capabilities improve, more patients are likely to be identified and treated, thereby increasing the demand for effective drugs. The market is expected to benefit from this trend, as pharmaceutical companies respond to the growing need for targeted therapies. Furthermore, the integration of advanced diagnostics into clinical practice may lead to better patient outcomes, reinforcing the importance of innovative drug development in the Pulmonary Alveolar Proteinosis Drug Market.
Growing Investment in Rare Disease Research
The growing investment in research focused on rare diseases, including Pulmonary Alveolar Proteinosis, is a critical driver for the Pulmonary Alveolar Proteinosis Drug Market. Governments and private organizations are increasingly allocating funds to support research initiatives aimed at understanding and treating rare conditions. This trend is particularly relevant for PAP, which has historically received limited attention. Increased funding is likely to accelerate the development of new therapies, as researchers explore novel treatment modalities. Additionally, collaborations between academic institutions and pharmaceutical companies are becoming more common, fostering innovation in drug development. As a result, the Pulmonary Alveolar Proteinosis Drug Market is poised for growth, with a pipeline of potential therapies that could address the needs of patients suffering from this rare disease.
Increased Awareness and Education Initiatives
Increased awareness and education initiatives surrounding Pulmonary Alveolar Proteinosis are playing a pivotal role in shaping the Pulmonary Alveolar Proteinosis Drug Market. Various organizations and advocacy groups are actively working to educate both healthcare professionals and the public about PAP. This heightened awareness is likely to lead to earlier diagnosis and treatment, thereby increasing the demand for effective therapies. Educational campaigns can also empower patients to seek medical attention, further driving market growth. As more individuals become informed about the symptoms and treatment options available, the market for PAP drugs is expected to expand. This trend underscores the importance of awareness in fostering a supportive environment for the development and adoption of new therapies within the Pulmonary Alveolar Proteinosis Drug Market.
Rising Incidence of Pulmonary Alveolar Proteinosis
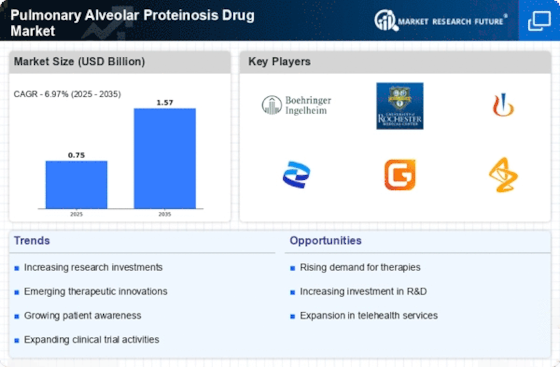
The increasing incidence of Pulmonary Alveolar Proteinosis (PAP) is a notable driver for the Pulmonary Alveolar Proteinosis Drug Market. Recent estimates suggest that PAP affects approximately 0.5 to 1.0 per 100,000 individuals annually. This rising prevalence is likely to spur demand for effective therapeutic options, thereby expanding the market. As awareness of PAP grows among healthcare professionals and patients, the need for specialized treatments becomes more pronounced. Consequently, pharmaceutical companies are motivated to invest in research and development, leading to the introduction of novel therapies. The growing patient population is expected to create a robust market environment, encouraging innovation and competition among drug manufacturers. This trend indicates a promising outlook for the Pulmonary Alveolar Proteinosis Drug Market, as stakeholders seek to address the unmet medical needs of affected individuals.