Emergence of Biologics and Biosimilars
The emergence of biologics and biosimilars is reshaping the North America Pharmacokinetics and Pharmacodynamics Market. Biologics, which are derived from living organisms, often exhibit complex pharmacokinetic and pharmacodynamic behaviors that necessitate specialized evaluation. The increasing prevalence of chronic diseases and the aging population are driving the demand for biologics, with the market for these therapies projected to exceed 200 billion USD by 2025. Additionally, the introduction of biosimilars is expected to enhance market competition, potentially lowering costs and improving patient access to essential therapies. As the landscape of biologics continues to evolve, the North America Pharmacokinetics and Pharmacodynamics Market is likely to witness substantial growth, fueled by innovations in drug formulation and delivery.
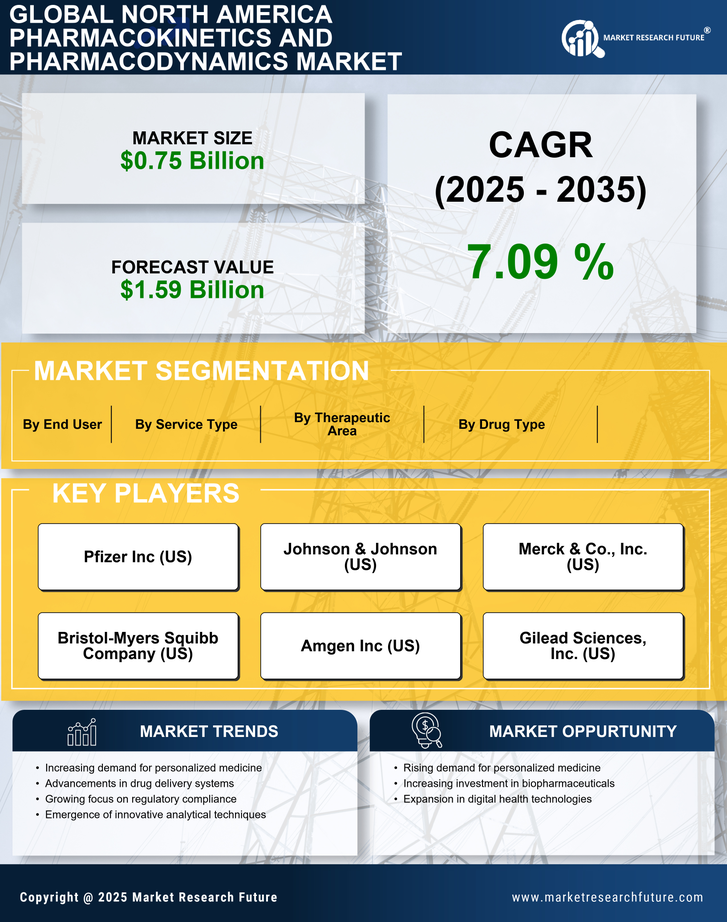
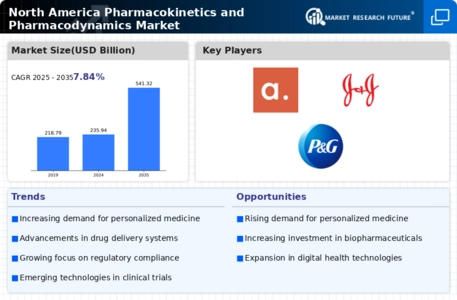
Rising Demand for Personalized Medicine
The North America Pharmacokinetics and Pharmacodynamics Market is experiencing a notable surge in demand for personalized medicine. This trend is driven by advancements in genomics and biotechnology, which enable tailored therapeutic approaches. As healthcare providers increasingly recognize the importance of individualized treatment plans, the market is projected to grow significantly. According to recent estimates, the personalized medicine sector is expected to reach a valuation of over 350 billion USD by 2025. This growth is indicative of a broader shift towards precision medicine, where pharmacokinetics and pharmacodynamics play a crucial role in optimizing drug efficacy and minimizing adverse effects. Consequently, the North America Pharmacokinetics and Pharmacodynamics Market is likely to benefit from this paradigm shift, as stakeholders seek to develop more effective and safer therapeutic options.
Growing Focus on Drug Safety and Efficacy
The emphasis on drug safety and efficacy is a driving force in the North America Pharmacokinetics and Pharmacodynamics Market. As healthcare providers and patients become increasingly aware of the potential risks associated with medications, there is a heightened demand for comprehensive pharmacokinetic and pharmacodynamic evaluations. This focus is particularly relevant in the context of chronic diseases, where long-term medication use necessitates rigorous safety assessments. Market data indicates that investments in pharmacovigilance and post-marketing studies are on the rise, reflecting a commitment to ensuring drug safety. Consequently, the North America Pharmacokinetics and Pharmacodynamics Market is likely to expand as stakeholders prioritize the development of safer and more effective therapeutic options.
Advancements in Drug Development Technologies
Technological innovations are reshaping the landscape of the North America Pharmacokinetics and Pharmacodynamics Market. The integration of advanced drug development technologies, such as high-throughput screening and in silico modeling, is enhancing the efficiency of drug discovery processes. These technologies facilitate the rapid assessment of pharmacokinetic and pharmacodynamic properties, thereby expediting the development of new therapeutics. Recent data suggests that the adoption of these technologies could reduce the time required for drug development by up to 30%. As pharmaceutical companies strive to bring new drugs to market more quickly, the North America Pharmacokinetics and Pharmacodynamics Market is poised for growth, driven by the need for innovative solutions that streamline the drug development pipeline.
Increasing Regulatory Support for Innovative Therapies
The North America Pharmacokinetics and Pharmacodynamics Market is benefiting from increasing regulatory support for innovative therapies. Regulatory agencies, such as the FDA, are actively promoting initiatives that facilitate the approval of novel drugs, particularly those that demonstrate unique pharmacokinetic and pharmacodynamic profiles. This supportive regulatory environment is crucial for the advancement of therapies targeting complex diseases, including cancer and rare genetic disorders. Recent reports indicate that the number of new drug approvals has risen significantly, with a notable increase in the approval of biologics and gene therapies. As regulatory frameworks continue to evolve, the North America Pharmacokinetics and Pharmacodynamics Market is likely to experience accelerated growth, as companies leverage these opportunities to introduce groundbreaking treatments.