Rising Incidence of Cancer
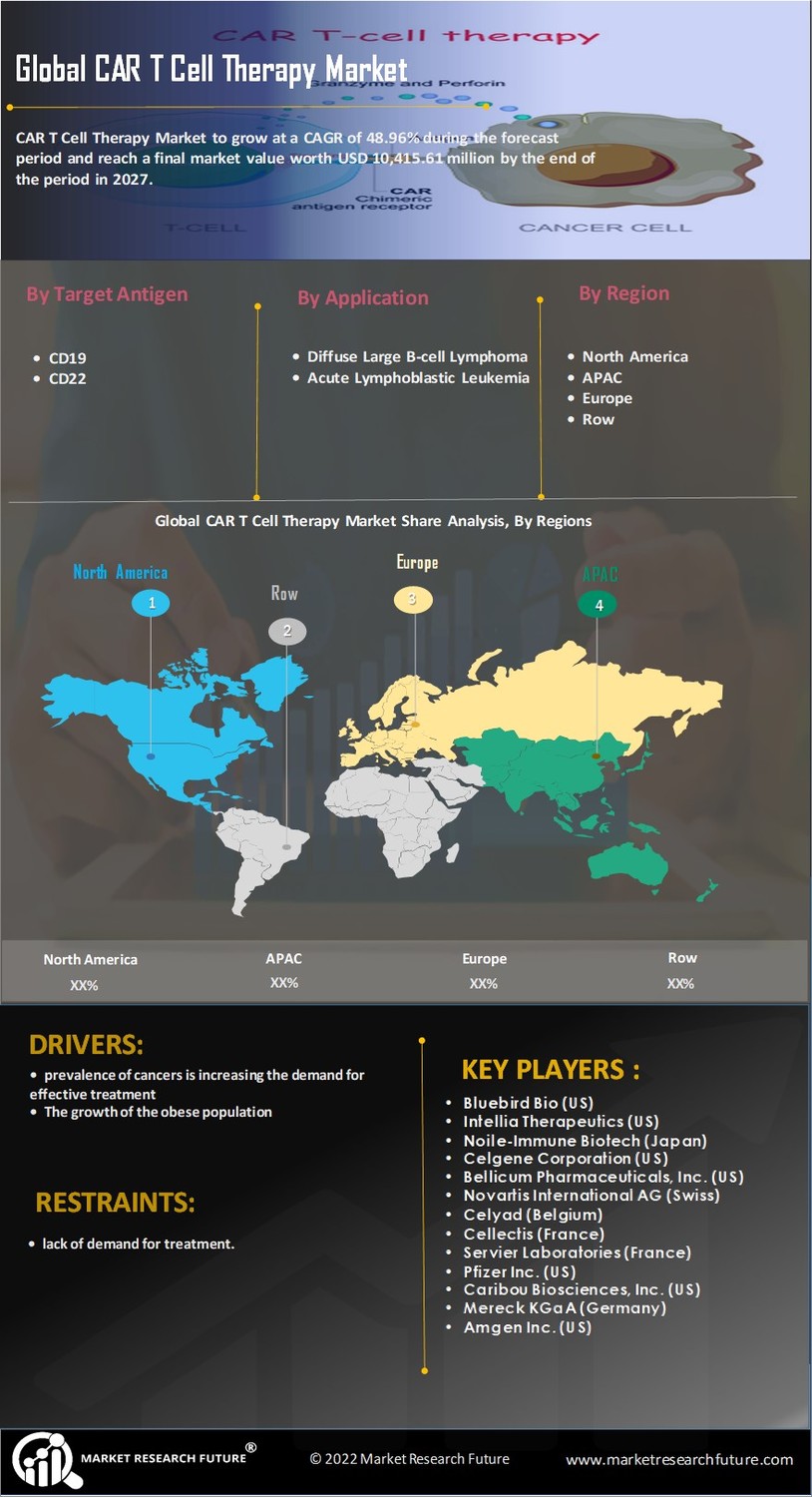
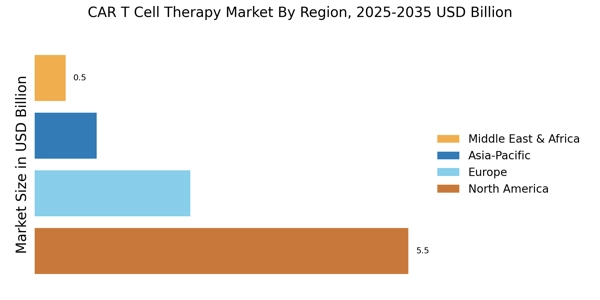
The increasing prevalence of various cancers is a primary driver for the CAR T Cell Therapy Market. As cancer cases rise, the demand for innovative treatment options intensifies. According to recent statistics, cancer is projected to affect millions annually, necessitating advanced therapies. CAR T cell therapy, known for its ability to target specific cancer cells, offers a promising solution. This therapy has shown remarkable efficacy in treating hematological malignancies, with response rates exceeding 80% in some cases. The growing patient population seeking effective treatments propels the CAR T Cell Therapy Market forward, as healthcare providers look for cutting-edge solutions to combat this escalating health crisis.
Growing Awareness and Acceptance
The growing awareness and acceptance of CAR T cell therapies among healthcare professionals and patients significantly impact the CAR T Cell Therapy Market. Educational initiatives and outreach programs have increased understanding of the benefits and potential of CAR T therapies. As more clinicians become familiar with these advanced treatments, the likelihood of their adoption in clinical practice rises. Additionally, patient advocacy groups play a vital role in promoting awareness, leading to increased demand for CAR T therapies. This heightened acceptance is likely to drive market growth, as more patients seek out these innovative options for their cancer treatment.
Regulatory Support and Approvals
Regulatory support plays a pivotal role in shaping the CAR T Cell Therapy Market. Regulatory agencies are increasingly recognizing the potential of CAR T therapies, expediting the approval process for new treatments. Recent approvals for several CAR T products have set a precedent, encouraging further development in this field. The streamlined regulatory pathways not only facilitate quicker access to therapies for patients but also instill confidence in investors and manufacturers. As more CAR T therapies gain regulatory approval, the market is expected to expand, providing patients with access to innovative treatment options that were previously unavailable.
Increasing Investment in Biotechnology
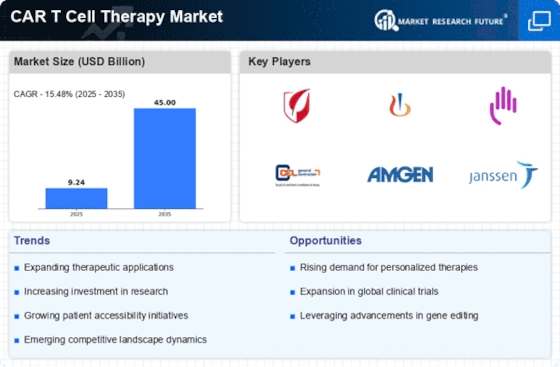
The surge in investment within the biotechnology sector is a crucial driver for the CAR T Cell Therapy Market. Venture capital and private equity funding have reached unprecedented levels, with billions directed towards companies specializing in CAR T cell therapies. This influx of capital facilitates the acceleration of clinical trials and the commercialization of new therapies. Furthermore, partnerships between biotech firms and academic institutions foster collaborative research, enhancing the development of innovative CAR T products. As financial backing continues to grow, the CAR T Cell Therapy Market is likely to witness rapid advancements, leading to a wider array of treatment options for patients.
Advancements in Research and Development
Ongoing advancements in research and development significantly influence the CAR T Cell Therapy Market. Continuous innovations in genetic engineering and cell processing technologies enhance the efficacy and safety profiles of CAR T therapies. Recent studies indicate that novel CAR constructs and combination therapies are being explored, potentially expanding the treatment landscape. The investment in R&D is substantial, with billions allocated to developing next-generation CAR T therapies. This focus on innovation not only improves patient outcomes but also attracts interest from pharmaceutical companies, thereby driving market growth. As new therapies emerge, the CAR T Cell Therapy Market is poised for expansion, catering to a broader range of malignancies.