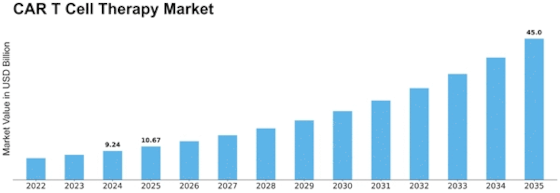
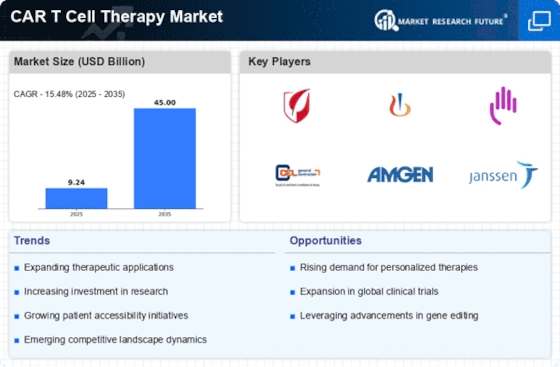
Car T Cell Therapy Size
CAR T Cell Therapy Market Growth Projections and Opportunities
Revolutionizing Cancer Treatment: A Deep Dive into CAR T Cell Therapy
In the realm of cancer treatment, Chimeric Antigen Receptor (CAR) T Cell Therapy stands as a beacon of hope, representing a groundbreaking immunotherapy. Let's delve into the intricacies of this therapy, its applications, and the factors propelling its growth on the global stage.
Understanding CAR T Cell Therapy: A Game-Changer in Cancer Treatment
CAR T Cell Therapy operates at the forefront of immunotherapy, offering a personalized approach to cancer treatment. The process involves isolating T cells from the patient's body and introducing the CAR gene. This genetic modification equips the T cells with a specific receptor that binds to a particular protein on the patient's cancer cells. This targeted approach enhances the body's ability to identify and eliminate cancer cells.
Driving Forces: Product Development and Clinical Studies
The market for CAR T Cell Therapy is set to soar, propelled by the surge in product development initiatives by various pharmaceutical and biotechnology companies. As these companies invest in creating innovative solutions for cancer treatment, the therapy's market share is anticipated to witness significant growth. The landscape is further enriched by a substantial increase in clinical studies within this domain. Researchers and healthcare professionals are exploring the potential of CAR T Cell Therapy across various cancer types, expanding its applications and refining its efficacy.
Global Prevalence of Cancer and FDA Approvals
The escalating prevalence of cancer worldwide is a pivotal factor bolstering the demand for advanced and effective treatment options. CAR T Cell Therapy, with its targeted and personalized approach, is emerging as a key player in the fight against cancer. The therapy's effectiveness is underscored by the growing number of approvals from the United States Food and Drug Administration (FDA) for cancer treatment therapies. These approvals validate the therapy's safety and efficacy, fostering increased adoption in clinical settings.
Market Dynamics: Growth Factors and Anticipated Expansion
The CAR T Cell Therapy market is poised for robust growth, fueled by a convergence of factors. The ongoing surge in product development efforts addresses the need for diverse and innovative solutions in cancer treatment. This commitment to innovation is reflected in the expanding scope of clinical studies, unraveling new possibilities for CAR T Cell Therapy applications.
The global landscape of cancer prevalence acts as a driving force, urging healthcare providers to explore and implement advanced treatment modalities. CAR T Cell Therapy, with its promise of personalized and targeted treatment, aligns seamlessly with this demand for more effective and tailored cancer therapies.
The string of FDA approvals further cements the therapy's standing in the market, instilling confidence in healthcare professionals and patients alike. As the therapy gains traction, its integration into standard cancer treatment protocols is becoming increasingly likely.
Challenges and Opportunities: Navigating the Future
While the prospects for CAR T Cell Therapy are bright, challenges persist. The intricate nature of genetic modifications and personalized treatments poses logistical and regulatory challenges. However, the industry's commitment to overcoming these obstacles, coupled with continuous advancements in gene editing technologies, signals a positive trajectory for CAR T Cell Therapy.
Opportunities abound in the evolving landscape of cancer treatment. The therapy's success stories, coupled with ongoing research and development, pave the way for a future where CAR T Cell Therapy becomes an integral part of the cancer treatment arsenal.
Conclusion: Paving the Way for Personalized Cancer Care
In conclusion, Chimeric Antigen Receptor (CAR) T Cell Therapy is not just a treatment; it represents a paradigm shift in the approach to cancer care. The convergence of product development, clinical studies, global cancer prevalence, and FDA approvals positions CAR T Cell Therapy as a transformative force in the field of oncology. As the therapy continues to evolve, it holds the promise of providing more patients with a personalized and targeted approach to cancer treatment, offering renewed hope and possibilities in the fight against this formidable disease.

















Leave a Comment