Rising Incidence of Uveitis
The increasing prevalence of non-infectious uveitis is a notable driver in the Non-Infectious Uveitis Market. Studies indicate that uveitis affects approximately 38 to 200 individuals per 100,000 people annually, with non-infectious forms accounting for a significant portion. This rise in incidence is attributed to various factors, including autoimmune diseases and systemic inflammatory conditions. As the population ages, the incidence of uveitis is expected to grow, leading to a heightened demand for effective treatment options. Consequently, pharmaceutical companies are likely to invest more in research and development, aiming to introduce innovative therapies that cater to this expanding patient population. This trend not only reflects the urgent need for effective management strategies but also underscores the potential for growth within the Non-Infectious Uveitis Market.
Advancements in Treatment Modalities
Innovations in treatment modalities are propelling the Non-Infectious Uveitis Market forward. The introduction of biologics and targeted therapies has transformed the management of non-infectious uveitis, offering patients more effective and personalized treatment options. For instance, biologics such as adalimumab and infliximab have shown promising results in clinical trials, leading to their increased adoption in clinical practice. Furthermore, the development of novel corticosteroid formulations and sustained-release delivery systems is enhancing therapeutic outcomes while minimizing side effects. As these advancements continue to emerge, they are likely to attract investment from pharmaceutical companies, thereby expanding the market landscape. The ongoing research into new therapeutic agents and treatment protocols suggests a dynamic evolution within the Non-Infectious Uveitis Market.
Regulatory Support for New Therapies
Regulatory support for the approval of new therapies is playing a pivotal role in shaping the Non-Infectious Uveitis Market. Regulatory agencies are increasingly recognizing the need for expedited review processes for innovative treatments that address unmet medical needs. This support is particularly relevant for biologics and novel drug formulations that demonstrate significant efficacy in clinical trials. The streamlined approval pathways not only facilitate quicker access to new therapies for patients but also encourage pharmaceutical companies to invest in the development of these treatments. As regulatory frameworks evolve to support innovation, the market for non-infectious uveitis therapies is likely to expand, providing patients with more options and improving overall treatment outcomes. This trend highlights the collaborative efforts between regulatory bodies and the pharmaceutical industry to enhance patient care.
Growing Awareness and Education Initiatives
The rise in awareness and education initiatives surrounding non-infectious uveitis is significantly influencing the Non-Infectious Uveitis Market. Organizations and healthcare providers are increasingly focusing on educating both patients and healthcare professionals about the symptoms, risks, and treatment options available for uveitis. This heightened awareness is likely to lead to earlier diagnosis and treatment, which can improve patient outcomes. Moreover, educational campaigns are fostering a better understanding of the disease, potentially increasing patient engagement in their treatment plans. As more individuals become informed about non-infectious uveitis, the demand for specialized care and innovative therapies is expected to rise, thereby driving growth in the market. This trend indicates a proactive approach to managing uveitis, which could reshape the landscape of the Non-Infectious Uveitis Market.
Increased Investment in Research and Development
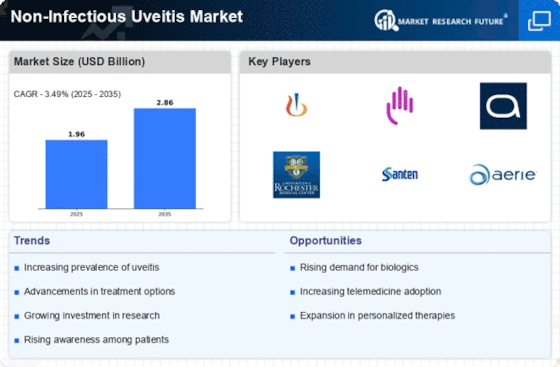
The surge in investment in research and development (R&D) is a critical driver for the Non-Infectious Uveitis Market. Pharmaceutical companies are recognizing the unmet needs in the treatment of non-infectious uveitis and are allocating substantial resources to develop new therapies. This investment is not only aimed at improving existing treatment options but also at discovering novel therapeutic targets. The global market for uveitis treatments is projected to reach several billion dollars by the end of the decade, reflecting the lucrative opportunities that lie within this sector. As R&D efforts intensify, the introduction of innovative therapies is expected to enhance patient care and expand the market. This trend underscores the commitment of the industry to address the challenges posed by non-infectious uveitis and improve treatment outcomes.