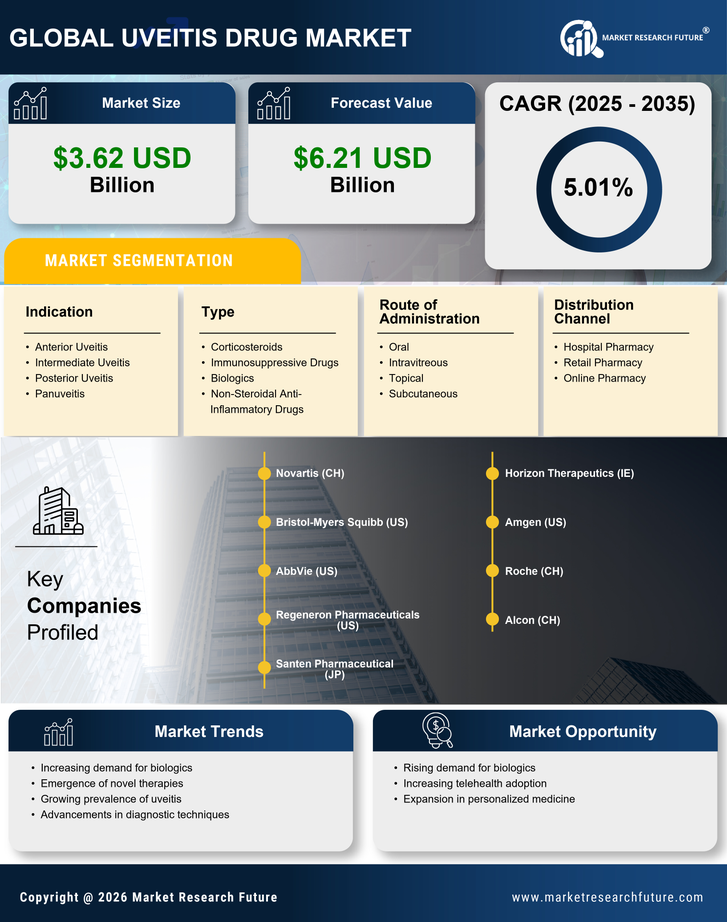
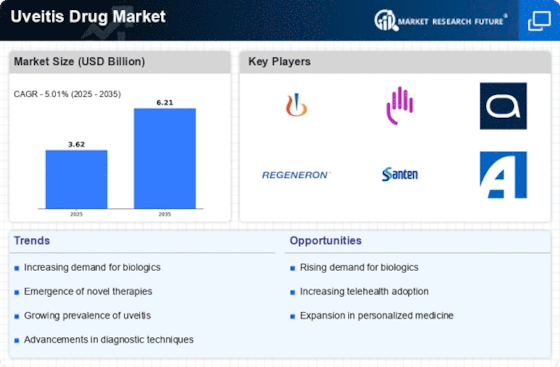
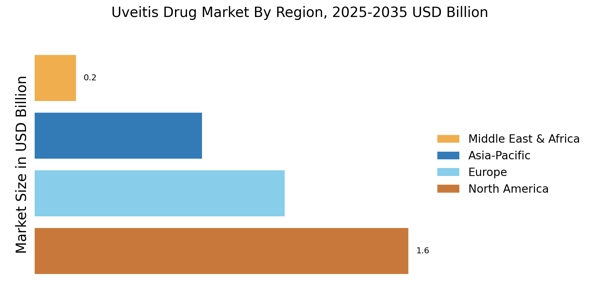
Rising Incidence of Uveitis
The Uveitis Drug Market is experiencing growth due to the increasing incidence of uveitis, a condition that affects the uvea of the eye. Recent studies indicate that uveitis affects approximately 38 to 200 individuals per 100,000 people annually, depending on the population studied. This rising prevalence is prompting healthcare providers to seek effective treatment options, thereby driving demand for uveitis drugs.
As awareness of the condition grows, more patients are being diagnosed and treated, which further stimulates the Uveitis Drug Market. The need for innovative therapies, particularly those that can address the underlying causes of uveitis, is becoming increasingly apparent. Consequently, pharmaceutical companies are investing in research and development to create new and effective medications, which is likely to enhance the overall market landscape.
Growing Awareness and Education
The Uveitis Drug Market is benefiting from growing awareness and education regarding uveitis among both healthcare professionals and patients. Increased educational initiatives, including workshops, seminars, and online resources, are helping to disseminate information about the symptoms, causes, and treatment options for uveitis. This heightened awareness is leading to earlier diagnosis and treatment, which is crucial for preventing vision loss associated with the condition.
Furthermore, patient advocacy groups are playing a pivotal role in promoting awareness and supporting research efforts. As more individuals become informed about uveitis, the demand for effective treatment options is likely to rise, thereby positively impacting the Uveitis Drug Market. The emphasis on education is expected to foster a more proactive approach to managing uveitis, ultimately benefiting patients and healthcare providers alike.
Advancements in Drug Development
The Uveitis Drug Market is significantly influenced by advancements in drug development technologies. Recent innovations in biologics and targeted therapies have opened new avenues for treating uveitis, which has historically been challenging to manage. The introduction of novel agents, such as corticosteroids and immunosuppressants, has shown promising results in clinical trials, leading to increased approval rates for new drugs. For instance, the approval of biologic agents has been associated with improved patient outcomes and reduced disease recurrence. This trend is likely to continue, as pharmaceutical companies are increasingly focusing on developing therapies that are not only effective but also have fewer side effects. The ongoing research in this area suggests a robust pipeline of potential treatments, which could further propel the Uveitis Drug Market in the coming years.
Increase in Healthcare Expenditure
The Uveitis Drug Market is poised for growth due to the increase in healthcare expenditure across various regions. As governments and private sectors allocate more funds towards healthcare, there is a corresponding rise in the availability of advanced treatment options for conditions like uveitis.
This increase in spending is facilitating the development and distribution of innovative drugs, which are essential for managing uveitis effectively. Moreover, higher healthcare budgets are enabling healthcare providers to invest in better diagnostic tools and treatment protocols, which can lead to improved patient outcomes. The trend of rising healthcare expenditure is likely to continue, driven by an aging population and the growing burden of chronic diseases, thereby providing a favorable environment for the Uveitis Drug Market to thrive.
Regulatory Support for Drug Approvals
The Uveitis Drug Market is experiencing a favorable regulatory environment that supports the approval of new drugs. Regulatory agencies are increasingly recognizing the need for effective treatments for uveitis, which has historically been underrepresented in the pharmaceutical landscape. Streamlined approval processes and incentives for developing orphan drugs are encouraging pharmaceutical companies to invest in uveitis therapies. This regulatory support is crucial for expediting the availability of new treatment options to patients. Additionally, the collaboration between regulatory bodies and industry stakeholders is fostering innovation and ensuring that new therapies meet safety and efficacy standards. As a result, the Uveitis Drug Market is likely to see a surge in new product launches, which could enhance competition and improve treatment outcomes for patients suffering from uveitis.