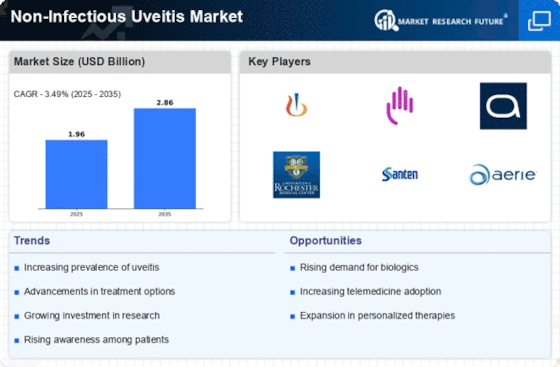
The Non-Infectious Uveitis Market is characterized by a dynamic competitive landscape, driven by increasing prevalence rates and a growing demand for effective therapeutic options. Key players such as Novartis (CH), Bristol-Myers Squibb (US), and AbbVie (US) are strategically positioned to leverage their extensive research and development capabilities. These companies focus on innovation and the introduction of novel therapies, which not only enhances their market presence but also contributes to the overall growth of the sector. The competitive environment is further shaped by partnerships and collaborations aimed at expanding product portfolios and enhancing market reach, indicating a trend towards cooperative strategies in addressing unmet medical needs.
In terms of business tactics, companies are increasingly localizing manufacturing and optimizing supply chains to enhance efficiency and reduce costs. The market structure appears moderately fragmented, with several players vying for market share. However, the collective influence of major companies like Roche (CH) and Santen Pharmaceutical (JP) suggests a trend towards consolidation, as these firms seek to strengthen their competitive positions through strategic acquisitions and collaborations.
In August 2025, Novartis (CH) announced a partnership with a leading biotechnology firm to co-develop a novel biologic therapy for non-infectious uveitis. This collaboration is expected to accelerate the development timeline and enhance the therapeutic options available to patients, reflecting Novartis's commitment to innovation and addressing the complexities of this condition. Such strategic alliances may also facilitate access to new technologies and expertise, thereby reinforcing Novartis's competitive edge in the market.
In September 2025, AbbVie (US) launched a new clinical trial for its investigational therapy targeting non-infectious uveitis, which is anticipated to provide a significant advancement in treatment efficacy. This move underscores AbbVie's focus on expanding its therapeutic offerings and demonstrates its proactive approach to addressing the evolving needs of patients. The trial's outcomes could potentially reshape treatment paradigms and solidify AbbVie's position as a leader in the uveitis space.
In July 2025, Aerie Pharmaceuticals (US) received regulatory approval for its latest product aimed at managing non-infectious uveitis, marking a pivotal moment in the company's growth trajectory. This approval not only enhances Aerie's product portfolio but also signifies a broader trend of regulatory bodies increasingly recognizing the need for innovative therapies in this area. The successful launch of this product could lead to increased market penetration and a stronger foothold in the competitive landscape.
As of October 2025, current trends in the Non-Infectious Uveitis Market indicate a shift towards digitalization, sustainability, and the integration of artificial intelligence in drug development processes. Strategic alliances are becoming increasingly vital, as companies recognize the importance of collaboration in navigating complex regulatory environments and accelerating innovation. Looking ahead, competitive differentiation is likely to evolve, with a pronounced emphasis on technological advancements and supply chain reliability, moving away from traditional price-based competition. This evolution suggests that companies that prioritize innovation and strategic partnerships will be better positioned to thrive in the future.