Advancements in Targeted Therapies
Innovations in targeted therapies represent a significant catalyst for the Mastocytosis Drug Market. Recent developments in molecular biology have led to the identification of specific genetic mutations associated with mastocytosis, paving the way for personalized treatment approaches. Targeted therapies, such as tyrosine kinase inhibitors, have shown promise in clinical trials, demonstrating improved efficacy and reduced side effects compared to traditional treatments. As these therapies gain regulatory approval, they are expected to capture a substantial share of the Mastocytosis Drug Market. The potential for combination therapies further enhances treatment outcomes, as clinicians seek to tailor interventions to individual patient profiles. This shift towards precision medicine is likely to reshape the therapeutic landscape for mastocytosis, fostering a competitive environment among pharmaceutical companies.
Increasing Prevalence of Mastocytosis
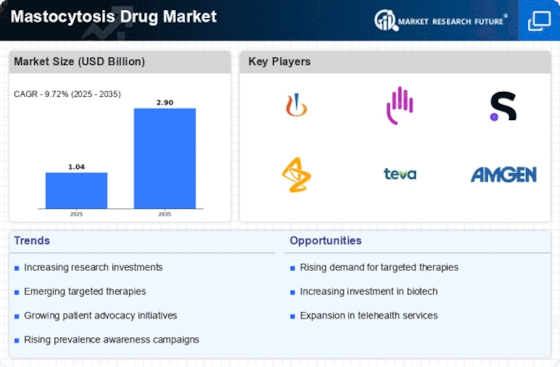
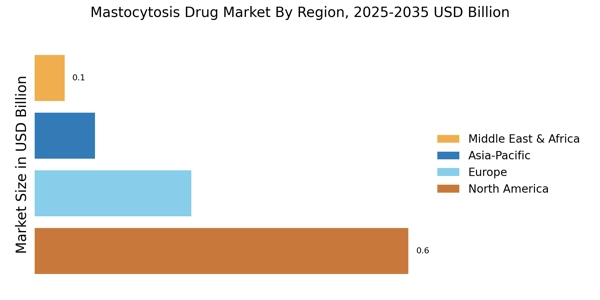
The rising incidence of mastocytosis is a pivotal driver for the Mastocytosis Drug Market. Recent estimates suggest that mastocytosis affects approximately 1 in 10,000 individuals, with a notable increase in diagnosed cases over the past decade. This trend is likely attributed to heightened awareness among healthcare professionals and advancements in diagnostic techniques. As more patients are identified, the demand for effective treatment options intensifies, propelling the growth of the Mastocytosis Drug Market. Furthermore, the increasing prevalence of associated comorbidities may complicate treatment regimens, necessitating a broader range of therapeutic solutions. Consequently, pharmaceutical companies are motivated to invest in research and development, aiming to address the unmet needs of this patient population.
Regulatory Support for New Treatments
Regulatory bodies are increasingly supportive of the development of new treatments for mastocytosis, which serves as a crucial driver for the Mastocytosis Drug Market. Recent initiatives aimed at expediting the approval process for orphan drugs have created a favorable environment for pharmaceutical companies. This regulatory landscape encourages innovation and facilitates the entry of novel therapies into the market. As a result, companies are more inclined to invest in the development of treatments specifically targeting mastocytosis. The potential for expedited review and approval timelines may lead to a quicker availability of effective therapies for patients. Furthermore, the establishment of clear guidelines for clinical trials enhances the feasibility of research efforts, ultimately benefiting the Mastocytosis Drug Market by ensuring that new treatments can reach patients in a timely manner.
Rising Patient Advocacy and Support Groups
The emergence of patient advocacy and support groups is playing an increasingly influential role in the Mastocytosis Drug Market. These organizations are dedicated to raising awareness about mastocytosis, providing education, and advocating for research funding. Their efforts contribute to a greater understanding of the disease among both the public and healthcare professionals, which may lead to earlier diagnosis and treatment. Additionally, these groups often collaborate with pharmaceutical companies to facilitate clinical trials and gather patient-reported outcomes, thereby enhancing the development of new therapies. As patient engagement continues to grow, it is likely that the demand for effective treatments will increase, further driving the Mastocytosis Drug Market. The collective voice of these advocacy groups may also influence policy decisions, ensuring that the needs of patients are prioritized in the healthcare landscape.
Growing Investment in Research and Development
The Mastocytosis Drug Market is experiencing a surge in investment directed towards research and development initiatives. Pharmaceutical companies are increasingly recognizing the need for novel treatment options, driven by the complexities of mastocytosis and its varied manifestations. Recent funding trends indicate a significant allocation of resources towards clinical trials and the exploration of new drug candidates. This influx of capital is expected to accelerate the pace of innovation, leading to the introduction of advanced therapies that address the diverse needs of patients. Moreover, collaborations between academic institutions and industry stakeholders are likely to enhance the research landscape, fostering a culture of innovation within the Mastocytosis Drug Market. As a result, the pipeline for new therapies appears robust, with several candidates poised to enter the market in the coming years.