Focus on Training and Education
A critical driver in the France emergency medical device services market industry is the emphasis on training and education for healthcare professionals. Continuous professional development ensures that medical personnel are well-versed in the latest emergency medical devices and protocols. The French government, alongside various healthcare organizations, has initiated programs aimed at enhancing the skills of emergency responders. These initiatives have resulted in improved patient care and outcomes, as trained personnel are more adept at utilizing advanced medical devices effectively. The investment in training not only elevates the standard of emergency medical services but also contributes to the overall growth of the France emergency medical device services market industry.
Regulatory Compliance and Innovation
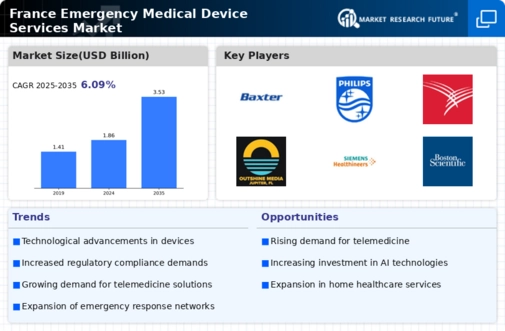
The France emergency medical device services market industry is significantly influenced by stringent regulatory frameworks that govern the approval and use of medical devices. The French government, through agencies such as the National Agency for the Safety of Medicines and Health Products (ANSM), enforces regulations that ensure the safety and efficacy of emergency medical devices. Compliance with these regulations not only fosters innovation but also enhances public trust in emergency medical services. As of 2025, the market has seen a rise in the introduction of innovative devices that meet these regulatory standards, indicating a robust environment for growth. The emphasis on compliance drives manufacturers to invest in research and development, thereby contributing to the overall advancement of the France emergency medical device services market industry.
Public Health Initiatives and Funding
Public health initiatives and government funding play a crucial role in shaping the France emergency medical device services market industry. The French government has allocated substantial resources towards enhancing emergency medical services, particularly in underserved areas. Initiatives aimed at improving access to emergency care and investing in state-of-the-art medical devices are indicative of a proactive approach to public health. For example, the recent funding increase for emergency medical services has facilitated the procurement of advanced life-saving devices, thereby improving service delivery. This commitment to public health not only enhances the capabilities of emergency services but also stimulates growth within the France emergency medical device services market industry.
Technological Integration in Emergency Services
The integration of advanced technologies into the France emergency medical device services market industry is a pivotal driver of growth. The adoption of telemedicine, mobile health applications, and real-time data analytics has transformed how emergency medical services operate. For instance, the use of mobile applications for dispatching emergency responders has improved response times significantly. According to recent data, the implementation of such technologies has led to a 20% increase in operational efficiency within emergency services. This technological evolution not only enhances patient outcomes but also streamlines the workflow of medical personnel, thereby reinforcing the importance of technology in the France emergency medical device services market industry.
Aging Population and Increased Demand for Emergency Services
The demographic shift towards an aging population in France is a significant driver of the emergency medical device services market industry. As the population ages, there is a corresponding increase in the prevalence of chronic diseases and emergencies requiring immediate medical attention. Data from the French National Institute of Statistics and Economic Studies (INSEE) indicates that by 2030, the proportion of individuals aged 65 and older will rise to 25% of the total population. This demographic trend is likely to escalate the demand for emergency medical services and, consequently, for advanced medical devices tailored to this age group. The growing need for efficient emergency response systems underscores the importance of the France emergency medical device services market industry.