Rising Healthcare Costs
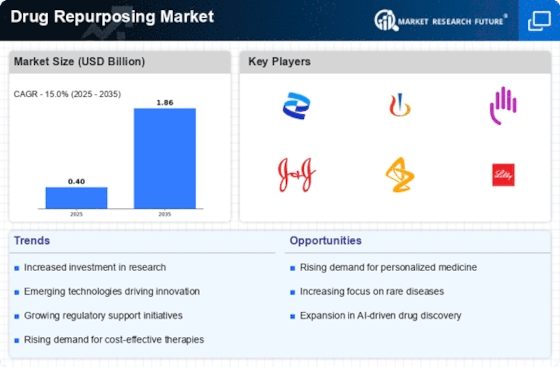
The escalating costs associated with healthcare are driving the Drug Repurposing Market. As traditional drug development can be prohibitively expensive, repurposing existing drugs offers a cost-effective alternative. It is estimated that the average cost of developing a new drug exceeds 2.6 billion USD, which has prompted pharmaceutical companies to explore repurposing strategies. This approach not only reduces the financial burden but also shortens the time to market, as repurposed drugs have already undergone extensive safety evaluations. Consequently, the Drug Repurposing Market is witnessing increased investment and interest from both public and private sectors, as stakeholders seek to optimize resources while addressing unmet medical needs.
Growing Prevalence of Chronic Diseases
The increasing incidence of chronic diseases such as diabetes, cancer, and cardiovascular disorders is significantly influencing the Drug Repurposing Market. With chronic diseases accounting for approximately 70% of all deaths worldwide, there is an urgent need for effective treatment options. Repurposing existing drugs can provide rapid solutions to these pressing health challenges. For instance, the repurposing of certain antidepressants for cancer treatment has shown promising results in clinical trials. This trend not only highlights the potential of existing medications but also emphasizes the necessity for innovative approaches in the Drug Repurposing Market to address the rising burden of chronic illnesses.
Regulatory Support for Drug Repurposing
Regulatory agencies are increasingly recognizing the value of drug repurposing, which is positively impacting the Drug Repurposing Market. Initiatives such as the FDA's 505(b)(2) application process facilitate the approval of repurposed drugs by allowing companies to leverage existing data. This regulatory support streamlines the pathway for bringing repurposed drugs to market, thereby encouraging pharmaceutical companies to invest in this area. The Drug Repurposing Market benefits from this favorable regulatory environment, as it reduces the time and resources required for drug approval, ultimately leading to faster access to therapies for patients.
Increased Collaboration Among Stakeholders
The Drug Repurposing Market is experiencing a surge in collaboration among various stakeholders, including academic institutions, pharmaceutical companies, and government agencies. These partnerships are essential for pooling resources, sharing knowledge, and accelerating the drug repurposing process. Collaborative initiatives often lead to the establishment of public-private partnerships that focus on specific therapeutic areas, thereby enhancing the efficiency of research efforts. This trend is particularly evident in the context of rare diseases, where collaboration can significantly impact the development of effective treatments. The Drug Repurposing Market is likely to benefit from these synergistic relationships, fostering innovation and expediting the delivery of new therapies to patients.
Advancements in Bioinformatics and Data Analytics
The rapid advancements in bioinformatics and data analytics are transforming the Drug Repurposing Market. These technologies enable researchers to analyze vast datasets, identify potential drug candidates, and predict their efficacy for new indications. Machine learning algorithms and artificial intelligence are increasingly employed to uncover hidden relationships between existing drugs and diseases. This data-driven approach not only accelerates the repurposing process but also enhances the precision of drug selection. As a result, the Drug Repurposing Market is poised for growth, driven by the integration of cutting-edge technologies that facilitate innovative research and development.