Advancements in Biobanking Technologies
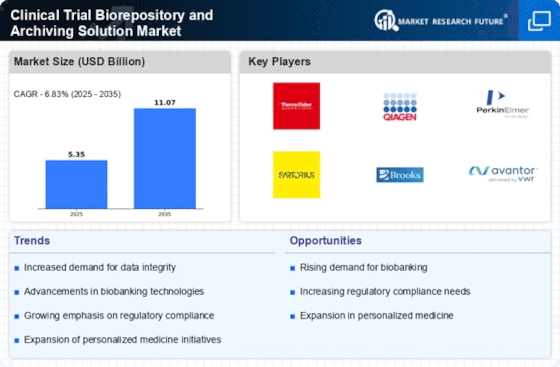
Technological advancements are significantly influencing the Clinical Trial Biorepository and Archiving Solution Market. Innovations in biobanking technologies, such as automated sample processing and advanced data management systems, are enhancing the efficiency and reliability of biorepositories. These advancements facilitate better sample tracking, storage conditions, and data integrity, which are critical for successful clinical trials. The integration of artificial intelligence and machine learning in biorepository operations is also emerging, potentially streamlining processes and improving decision-making. As a result, organizations are increasingly investing in state-of-the-art biorepository solutions to maintain a competitive edge in the evolving landscape of clinical research.
Growing Investment in Clinical Research
The Clinical Trial Biorepository and Archiving Solution Market is benefiting from a growing investment in clinical research. As the pharmaceutical and biotechnology sectors expand their research portfolios, the need for efficient biorepository solutions becomes increasingly apparent. Recent statistics indicate that global spending on clinical trials is on an upward trajectory, which is likely to drive demand for biorepositories that can support large-scale studies. This influx of investment not only enhances the capabilities of biorepositories but also fosters collaborations between research institutions and biopharmaceutical companies. As a result, the market for biorepository solutions is poised for growth, reflecting the increasing importance of well-managed biological sample collections in clinical research.
Rising Demand for Personalized Medicine
The Clinical Trial Biorepository and Archiving Solution Market is experiencing a notable surge in demand for personalized medicine. This trend is driven by the increasing recognition of the need for tailored therapies that cater to individual patient profiles. As biorepositories play a crucial role in storing biological samples for genetic and biomarker research, their importance in clinical trials is amplified. According to recent data, the market for personalized medicine is projected to reach substantial figures, indicating a robust growth trajectory. This growth is likely to propel investments in biorepository solutions, as pharmaceutical companies seek to enhance their research capabilities and improve patient outcomes through more precise treatment options.
Increased Focus on Regulatory Compliance
Regulatory compliance remains a pivotal driver in the Clinical Trial Biorepository and Archiving Solution Market. With stringent regulations governing the handling and storage of biological samples, organizations are compelled to adopt robust biorepository solutions that ensure adherence to these standards. Regulatory bodies are continuously updating guidelines, which necessitates that biorepositories implement comprehensive quality management systems. This focus on compliance not only mitigates risks associated with non-compliance but also enhances the credibility of clinical trials. Consequently, the demand for biorepository solutions that facilitate regulatory adherence is expected to rise, as organizations strive to maintain operational integrity and uphold public trust.
Emergence of Collaborative Research Models
The emergence of collaborative research models is reshaping the Clinical Trial Biorepository and Archiving Solution Market. As research becomes more interdisciplinary, the need for shared biorepository resources is growing. Collaborative initiatives among academic institutions, healthcare organizations, and industry players are fostering the pooling of biological samples and data, which enhances the quality and scope of clinical trials. This trend is likely to lead to the establishment of centralized biorepositories that serve multiple stakeholders, thereby optimizing resource utilization. The shift towards collaboration not only accelerates research timelines but also promotes innovation in clinical trial methodologies, further driving the demand for sophisticated biorepository solutions.