
Clinical Trial Biorepository Archiving Solution Market
Rapport d’étude de marché sur les solutions d’archivage et de bioréférentiel d’essais cliniques par application (recherche et développement, essais cliniques, conformité réglementaire, développement de médicaments), par type d’échantillon (échantillons biologiques, stockage pharmaceutique, données cliniques, données génomiques), par utilisateur final (sociétés pharmaceutiques, entreprises de biotechnologie, organismes de recherche sous contrat, établissements universitaires), par type de stockage (entreposage frigorifique, stockage à température ambiante, stockage au congélateur, stockage cryogénique) et par région (Amérique du Nord, Europe, Amérique du Sud, Asie-Pacifique, Moyen-Orient et Afrique) - Prévisions jusqu'en 2034
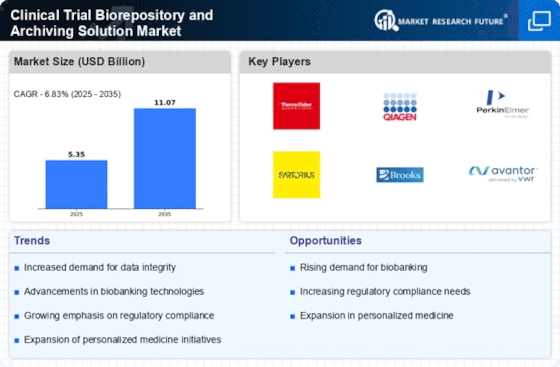
Les principales entreprises du marché Clinical Trial Biorepository Archiving Solution Market incluent






Portée du rapport
FAQs
What is the projected market valuation for the Clinical Trial Biorepository and Archiving Solution Market in 2035?
The projected market valuation for the Clinical Trial Biorepository and Archiving Solution Market in 2035 is 11.07 USD Billion.
What was the overall market valuation in 2024?
The overall market valuation for the Clinical Trial Biorepository and Archiving Solution Market was 5.353 USD Billion in 2024.
What is the expected CAGR for the market during the forecast period 2025 - 2035?
The expected CAGR for the Clinical Trial Biorepository and Archiving Solution Market during the forecast period 2025 - 2035 is 6.83%.
Which companies are considered key players in the Clinical Trial Biorepository and Archiving Solution Market?
Key players in the market include Thermo Fisher Scientific, Qiagen, PerkinElmer, Sartorius, Brooks Automation, VWR, Labcorp, Celerion, and Charles River Laboratories.
What are the main application segments of the Clinical Trial Biorepository and Archiving Solution Market?
The main application segments include Research and Development, Clinical Trials, Regulatory Compliance, and Drug Development, with valuations ranging from 1.0 to 3.37 USD Billion.
How does the market perform in terms of sample types?
In terms of sample types, Biological Samples and Genomic Data show valuations from 1.5 to 3.17 USD Billion, indicating robust growth potential.
What are the storage types utilized in the Clinical Trial Biorepository and Archiving Solution Market?
Storage types include Cold Storage, Room Temperature Storage, Deep Freezer Storage, and Cryogenic Storage, with valuations between 1.0 and 3.1 USD Billion.
Which end users are driving the Clinical Trial Biorepository and Archiving Solution Market?
End users driving the market include Pharmaceutical Companies, Biotechnology Firms, Contract Research Organizations, and Academic Institutions, with valuations from 0.653 to 5.2 USD Billion.
What trends are observed in the Clinical Trial Biorepository and Archiving Solution Market?
Trends indicate a growing emphasis on regulatory compliance and data integrity, as evidenced by the increasing valuations across various segments.
How does the market's growth trajectory appear for the next decade?
The market's growth trajectory appears promising, with a projected increase in valuation and a consistent CAGR of 6.83% anticipated through 2035.
Veuillez remplir le formulaire ci-dessous pour recevoir un échantillon gratuit de ce rapport
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”
