Innovations in Gene Therapy
Innovations in gene therapy represent a transformative force within the Choroideremia Treatment Market. Recent advancements have led to the development of novel therapeutic approaches aimed at addressing the underlying genetic causes of choroideremia. For instance, gene replacement therapies are being explored, which could potentially restore function to the defective gene responsible for the condition. The success of early clinical trials has generated optimism among researchers and investors alike, suggesting a promising future for these therapies. As the scientific community continues to refine these techniques, the Choroideremia Treatment Market may witness a surge in new product offerings, enhancing treatment options for patients. This innovation-driven landscape is likely to attract further investment, thereby accelerating the pace of development and commercialization of effective therapies.
Growing Patient Advocacy Groups
The emergence of growing patient advocacy groups is influencing the Choroideremia Treatment Market in a profound manner. These organizations play a pivotal role in raising awareness about choroideremia, advocating for research funding, and supporting affected individuals and their families. By mobilizing communities and fostering collaboration among stakeholders, patient advocacy groups are driving demand for effective treatments. Their efforts to educate the public and healthcare professionals about the condition are likely to lead to earlier diagnoses and increased treatment uptake. Furthermore, these groups often collaborate with pharmaceutical companies to ensure that patient perspectives are integrated into the development of new therapies. As a result, the Choroideremia Treatment Market is experiencing a shift towards more patient-centered approaches, which may enhance the overall effectiveness of treatment strategies.
Rising Prevalence of Choroideremia
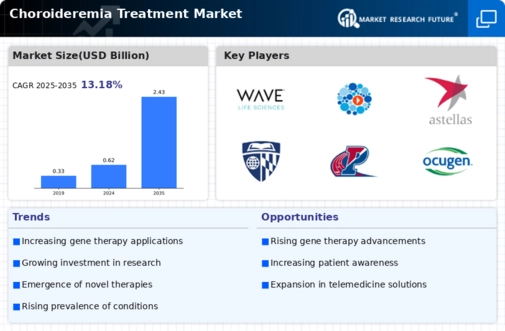
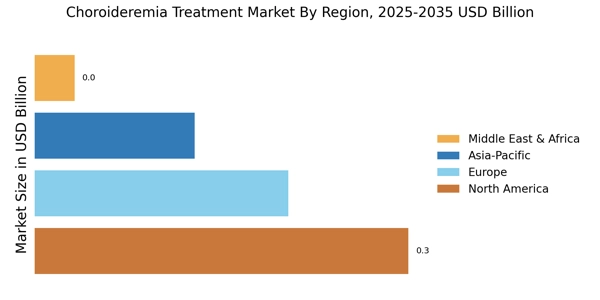
The increasing prevalence of choroideremia is a notable driver for the Choroideremia Treatment Market. As awareness of this rare genetic disorder grows, more individuals are being diagnosed, leading to a heightened demand for effective treatment options. Recent estimates suggest that choroideremia affects approximately 1 in 50,000 males, indicating a significant patient population that requires specialized care. This rising incidence is prompting pharmaceutical companies to invest in research and development, thereby expanding the treatment landscape. The growing number of diagnosed cases is likely to stimulate market growth, as healthcare providers seek innovative therapies to address the needs of affected individuals. Consequently, the Choroideremia Treatment Market is poised for expansion as stakeholders respond to the increasing demand for effective interventions.
Increased Funding for Rare Disease Research
Increased funding for rare disease research is a critical driver for the Choroideremia Treatment Market. Governments and private organizations are recognizing the need to allocate resources towards understanding and treating rare genetic disorders like choroideremia. This financial support is facilitating the advancement of research initiatives, clinical trials, and the development of new therapies. For instance, funding from national health agencies has been directed towards projects aimed at exploring innovative treatment modalities. As a result, the Choroideremia Treatment Market is likely to benefit from a more robust pipeline of potential therapies, which could lead to improved patient outcomes. The influx of capital into this sector underscores the commitment to addressing the unmet medical needs of individuals affected by choroideremia.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is emerging as a significant driver within the Choroideremia Treatment Market. Regulatory agencies are increasingly recognizing the need for expedited pathways for the approval of treatments targeting rare diseases. This supportive environment encourages pharmaceutical companies to invest in the development of novel therapies for choroideremia. Initiatives such as orphan drug designations and fast-track approvals are designed to facilitate the timely availability of new treatments to patients. As a result, the Choroideremia Treatment Market is likely to see a more rapid introduction of innovative therapies, which could address the urgent needs of affected individuals. This regulatory landscape not only fosters innovation but also enhances the overall competitiveness of the market.