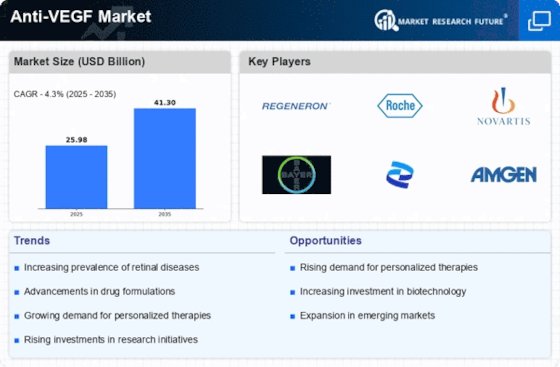
Rising Incidence of Eye Diseases
The increasing prevalence of eye diseases, particularly age-related macular degeneration (AMD) and diabetic retinopathy, is a primary driver of the Anti-VEGF Market. As populations age, the incidence of these conditions rises, leading to a greater demand for effective treatments. According to recent estimates, AMD affects millions worldwide, with projections indicating that the number of affected individuals will continue to grow. This trend necessitates the development and availability of Anti-VEGF therapies, which have proven effective in managing these diseases. Consequently, pharmaceutical companies are investing heavily in research and development to introduce innovative Anti-VEGF products, thereby expanding the market. The rising incidence of eye diseases not only highlights the need for effective treatment options but also underscores the potential for growth within the Anti-VEGF Market.
Advancements in Drug Formulations
Innovations in drug formulations are significantly influencing the Anti-VEGF Market. The development of long-acting formulations and sustained-release delivery systems enhances the efficacy and convenience of Anti-VEGF therapies. For instance, recent advancements have led to the creation of injectable formulations that require less frequent administration, improving patient compliance. This is particularly relevant in the treatment of chronic conditions like AMD, where consistent therapy is crucial for maintaining vision. Furthermore, the introduction of combination therapies that integrate Anti-VEGF agents with other treatment modalities is gaining traction. These advancements not only improve therapeutic outcomes but also expand the market potential for Anti-VEGF products, as healthcare providers seek more effective and patient-friendly treatment options.
Increasing Healthcare Expenditure
The rise in healthcare expenditure across various regions is a significant driver for the Anti-VEGF Market. As governments and private sectors allocate more resources to healthcare, there is a corresponding increase in the availability and accessibility of advanced medical treatments, including Anti-VEGF therapies. Reports indicate that healthcare spending is projected to grow, driven by the need to address chronic diseases and improve patient outcomes. This trend is particularly evident in developed economies, where healthcare systems are increasingly adopting innovative therapies to enhance treatment efficacy. The increased funding allows for better access to Anti-VEGF treatments, thereby fostering market growth. As healthcare expenditure continues to rise, the Anti-VEGF Market is likely to benefit from enhanced investment in research, development, and distribution of these critical therapies.
Growing Awareness and Screening Programs
The growing awareness of eye health and the implementation of screening programs are pivotal in driving the Anti-VEGF Market. Public health initiatives aimed at educating populations about the importance of early detection and treatment of eye diseases have led to increased screening rates. As more individuals undergo regular eye examinations, the identification of conditions such as AMD and diabetic retinopathy has become more prevalent. This heightened awareness not only facilitates early intervention but also drives demand for Anti-VEGF therapies as effective treatment options. Moreover, healthcare providers are increasingly advocating for routine screenings, further propelling the market. The synergy between public health efforts and the Anti-VEGF Market is likely to result in sustained growth as more patients seek timely and effective treatments.
Regulatory Approvals and Market Entry of New Products
The landscape of the Anti-VEGF Market is being shaped by the regulatory approvals and the entry of new products. Regulatory agencies are increasingly expediting the approval processes for innovative Anti-VEGF therapies, recognizing the urgent need for effective treatments in managing eye diseases. This trend is evident in the recent approvals of novel Anti-VEGF agents that offer improved efficacy and safety profiles. The introduction of these new products not only enhances treatment options for healthcare providers but also stimulates competition within the market. As more companies seek to enter the Anti-VEGF Market with innovative solutions, the overall market is likely to expand. The dynamic nature of regulatory frameworks and the continuous influx of new therapies are expected to drive growth and innovation in the Anti-VEGF Market.