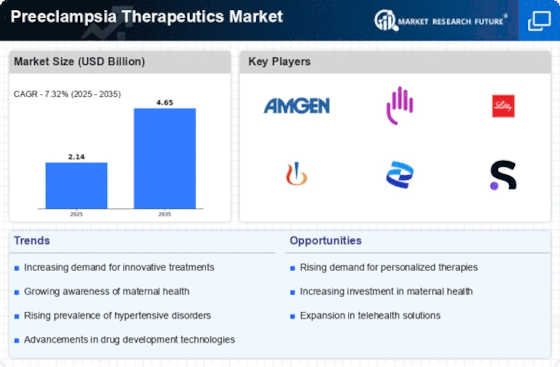
The Preeclampsia Therapeutics Market is currently characterized by a dynamic competitive landscape, driven by increasing awareness of maternal health and the rising incidence of preeclampsia globally. Key players such as Amgen (US), Bristol-Myers Squibb (US), and Eli Lilly and Company (US) are strategically positioned to leverage innovation and partnerships to enhance their market presence. Amgen (US) focuses on developing novel therapeutics that target the underlying mechanisms of preeclampsia, while Bristol-Myers Squibb (US) emphasizes strategic collaborations with research institutions to accelerate drug development. Eli Lilly and Company (US) is actively pursuing regional expansion, particularly in emerging markets, to tap into the growing demand for effective preeclampsia treatments. Collectively, these strategies contribute to a competitive environment that is increasingly centered on innovation and collaborative efforts.
In terms of business tactics, companies are localizing manufacturing and optimizing supply chains to enhance efficiency and reduce costs. The market structure appears moderately fragmented, with several players vying for market share. However, the collective influence of major companies like Novartis (CH) and Pfizer (US) is notable, as they continue to invest in research and development to bring new therapies to market. This competitive structure fosters an environment where innovation is paramount, and companies are compelled to differentiate themselves through unique offerings.
In August 2025, Novartis (CH) announced a strategic partnership with a leading biotechnology firm to co-develop a novel therapeutic agent aimed at reducing the incidence of preeclampsia. This collaboration is significant as it not only enhances Novartis's research capabilities but also positions the company to potentially lead in a niche segment of the market. The partnership underscores the importance of collaborative innovation in addressing complex health challenges associated with preeclampsia.
In September 2025, Pfizer (US) launched a new clinical trial for a promising drug candidate that targets the vascular complications associated with preeclampsia. This initiative is crucial as it reflects Pfizer's commitment to advancing treatment options and addressing unmet medical needs. The trial's outcomes could significantly influence the company's market positioning and contribute to a broader understanding of preeclampsia therapeutics.
In October 2025, Eli Lilly and Company (US) expanded its global footprint by entering into a distribution agreement with a regional healthcare provider in Southeast Asia. This move is indicative of Eli Lilly's strategy to enhance accessibility to its therapies in underserved markets. By establishing a presence in this region, the company aims to capture a larger share of the growing demand for preeclampsia treatments, thereby reinforcing its competitive stance.
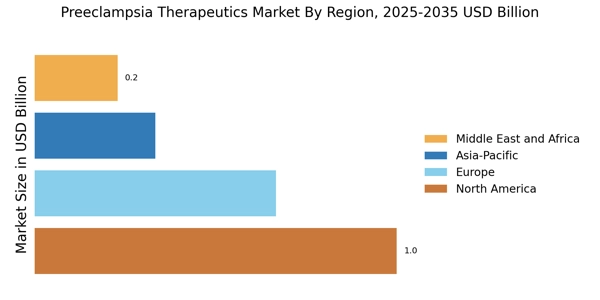
As of October 2025, current trends in the Preeclampsia Therapeutics Market are increasingly defined by digitalization, sustainability, and the integration of artificial intelligence in drug development processes. Strategic alliances are shaping the landscape, enabling companies to pool resources and expertise to accelerate innovation. Looking ahead, competitive differentiation is likely to evolve from traditional price-based competition to a focus on technological advancements, innovative therapies, and reliable supply chains. This shift may ultimately enhance patient outcomes and drive growth in the Preeclampsia Therapeutics Market.
















发表评论