Increasing Incidence of Preeclampsia
The rising incidence of preeclampsia is a notable driver in the Preeclampsia Therapeutics Market. Recent data indicates that the prevalence of preeclampsia has been increasing, with estimates suggesting that it affects approximately 5-8% of pregnancies. This uptick in cases necessitates the development and availability of effective therapeutics, thereby propelling market growth. As healthcare providers become more vigilant in diagnosing and managing this condition, the demand for innovative treatment options is likely to rise. Furthermore, the increasing awareness among expectant mothers regarding the risks associated with preeclampsia contributes to a heightened demand for therapeutics. This trend underscores the importance of addressing preeclampsia through targeted therapies, which could potentially enhance maternal and fetal outcomes.
Regulatory Support for New Therapies
Regulatory support for new therapies is emerging as a significant driver in the Preeclampsia Therapeutics Market. Regulatory agencies are increasingly streamlining the approval processes for innovative treatments, which is likely to encourage pharmaceutical companies to invest in the development of new therapeutics. This supportive environment fosters a climate of innovation, enabling faster access to potentially life-saving therapies for preeclampsia. Moreover, the establishment of expedited pathways for drug approval can significantly reduce the time it takes for new treatments to reach the market. As a result, the therapeutic options available for managing preeclampsia are expected to diversify, catering to the needs of healthcare providers and patients alike. This trend not only enhances the market landscape but also underscores the commitment of regulatory bodies to improve maternal health outcomes.
Growing Investment in Maternal Health
Growing investment in maternal health initiatives is a critical driver for the Preeclampsia Therapeutics Market. Governments and private organizations are increasingly recognizing the importance of maternal health, leading to enhanced funding for research and development of therapeutics. This investment is aimed at addressing the challenges posed by conditions like preeclampsia, which can have severe implications for both mothers and infants. The financial support directed towards innovative treatment options is likely to foster a more robust pipeline of therapeutics, thereby expanding market opportunities. Furthermore, initiatives aimed at improving healthcare infrastructure and access to maternal care are expected to contribute to the overall growth of the market. As a result, the therapeutic landscape for preeclampsia is poised for transformation, with a focus on developing effective solutions to mitigate risks associated with this condition.
Rising Focus on Personalized Medicine
Rising focus on personalized medicine is shaping the Preeclampsia Therapeutics Market. The shift towards tailored treatment approaches is becoming increasingly prevalent, as healthcare providers recognize that preeclampsia can manifest differently among individuals. This understanding is driving research into personalized therapeutics that consider genetic, environmental, and lifestyle factors. The potential for developing targeted therapies that address the unique needs of patients with preeclampsia is likely to enhance treatment efficacy and improve outcomes. Furthermore, the integration of genomic data into clinical practice may facilitate the identification of at-risk populations, allowing for proactive management strategies. As personalized medicine continues to gain traction, the market for preeclampsia therapeutics is expected to evolve, offering more effective and individualized treatment options.
Advancements in Diagnostic Technologies
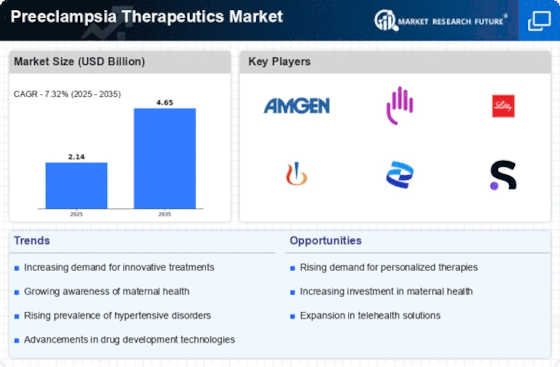
Advancements in diagnostic technologies are significantly influencing the Preeclampsia Therapeutics Market. Enhanced diagnostic tools, such as biomarkers and imaging techniques, facilitate earlier detection of preeclampsia, allowing for timely intervention. The integration of these technologies into clinical practice is likely to lead to an increase in the number of diagnosed cases, thereby driving demand for therapeutics. For instance, the development of point-of-care testing devices enables healthcare providers to assess risk factors more efficiently. As a result, the market for preeclampsia therapeutics is expected to expand, with a projected growth rate of around 7% annually over the next few years. This growth is indicative of the increasing reliance on advanced diagnostics to inform treatment decisions and improve patient outcomes.