Rising Demand for Vaccines
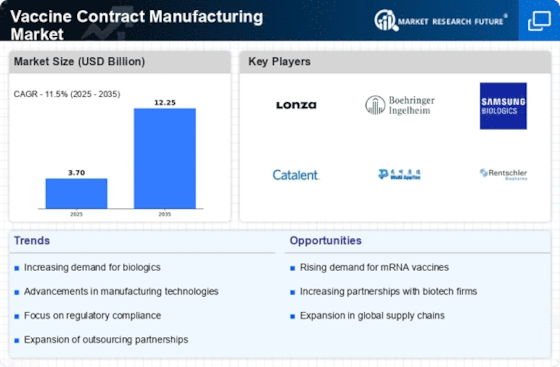
The Vaccine Contract Manufacturing Market is experiencing a notable increase in demand for vaccines, driven by the growing awareness of preventive healthcare. As populations become more health-conscious, the need for vaccines to combat infectious diseases is escalating. According to recent data, the vaccine market is projected to reach approximately USD 60 billion by 2025, indicating a robust growth trajectory. This surge in demand compels manufacturers to seek contract manufacturing services to scale production efficiently. The Vaccine Contract Manufacturing Market thus benefits from this trend, as companies look to outsource production to meet the rising needs of healthcare providers and governments. Furthermore, the increasing prevalence of vaccine-preventable diseases underscores the necessity for reliable manufacturing partners, enhancing the attractiveness of the contract manufacturing model.
Regulatory Support and Compliance
Regulatory frameworks are evolving to support the Vaccine Contract Manufacturing Market, facilitating smoother pathways for vaccine production and distribution. Governments are recognizing the importance of vaccines in public health and are streamlining regulatory processes to expedite approvals. This supportive environment encourages manufacturers to engage in contract manufacturing, as compliance with regulations becomes more manageable. The Vaccine Contract Manufacturing Market is likely to see increased participation from new entrants, as the barriers to entry are lowered. Moreover, regulatory agencies are providing guidance on best practices, which enhances the overall quality and safety of vaccine production. As a result, the alignment of regulatory support with industry needs is expected to drive growth in the Vaccine Contract Manufacturing Market, fostering innovation and collaboration among stakeholders.
Increasing Focus on Vaccine Development
The Vaccine Contract Manufacturing Market is being propelled by an increasing focus on vaccine development, particularly in response to emerging infectious diseases. Governments and private entities are investing heavily in research and development to create new vaccines, which necessitates robust manufacturing capabilities. The market for vaccine development is projected to grow significantly, with estimates suggesting a compound annual growth rate of over 10% in the coming years. This trend creates opportunities for contract manufacturers to collaborate with biotech firms and pharmaceutical companies, providing the necessary infrastructure and expertise to bring new vaccines to market efficiently. As the urgency for innovative vaccine solutions rises, the Vaccine Contract Manufacturing Market stands to benefit from these collaborative efforts, enhancing its role in the broader healthcare ecosystem.
Cost Efficiency and Resource Optimization
In the Vaccine Contract Manufacturing Market, cost efficiency plays a pivotal role in driving growth. Many pharmaceutical companies are opting for contract manufacturing to reduce operational costs associated with in-house production. By outsourcing vaccine production, companies can allocate resources more effectively, focusing on research and development while leveraging the expertise of specialized manufacturers. This approach not only minimizes capital expenditure but also enhances flexibility in production capacity. Data suggests that companies utilizing contract manufacturing can save up to 30% in production costs, making it an appealing option. As the industry evolves, the emphasis on cost-effective solutions is likely to propel the Vaccine Contract Manufacturing Market further, as firms seek to maintain competitive pricing while ensuring high-quality vaccine production.
Technological Innovations in Manufacturing
Technological advancements are significantly influencing the Vaccine Contract Manufacturing Market. The integration of cutting-edge technologies such as automation, artificial intelligence, and advanced bioprocessing techniques is enhancing production efficiency and quality. These innovations enable manufacturers to streamline operations, reduce lead times, and improve yield rates. For instance, the adoption of continuous manufacturing processes is transforming traditional batch production methods, allowing for more agile responses to market demands. As a result, the Vaccine Contract Manufacturing Market is likely to witness increased investment in technology-driven solutions, fostering a more competitive landscape. Furthermore, the ability to produce vaccines at a larger scale with consistent quality is becoming a critical factor for success, making technological innovation a key driver in this sector.