Growing Focus on Cost Efficiency
Cost efficiency is becoming a critical driver in the US Medical Sector Contract Electronic Manufacturing Market. As healthcare costs continue to rise, medical device companies are under pressure to reduce expenses while maintaining high-quality standards. Contract manufacturers are responding by optimizing their supply chains and leveraging economies of scale to offer competitive pricing. This trend is evident in the increasing number of partnerships between medical firms and contract manufacturers, as companies seek to streamline operations and focus on core competencies. By outsourcing electronic manufacturing, medical device companies can allocate resources more effectively, ultimately enhancing their market position and profitability.
Rising Demand for Medical Devices
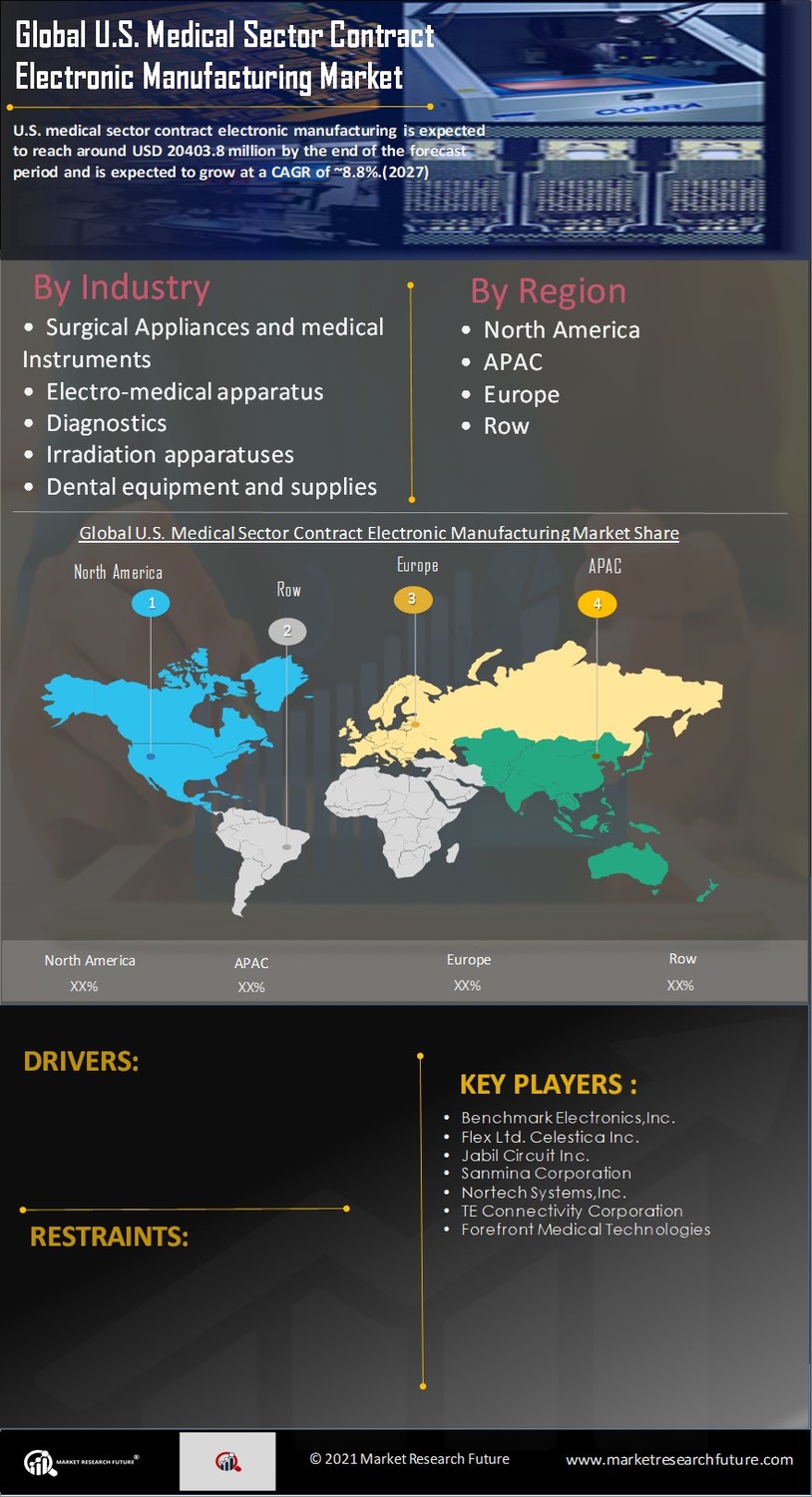
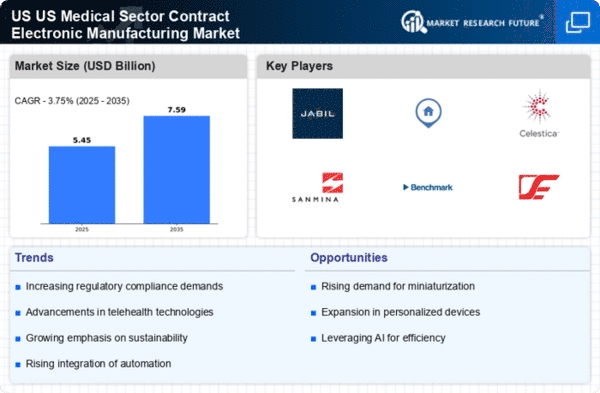
The US Medical Sector Contract Electronic Manufacturing Market is experiencing a notable increase in demand for medical devices. This surge is driven by an aging population and a growing prevalence of chronic diseases, which necessitate advanced medical technologies. According to recent data, the medical device market in the US is projected to reach approximately 208 billion USD by 2026. This growth creates opportunities for contract manufacturers to provide specialized electronic components and assemblies tailored to the needs of medical device companies. As healthcare providers seek innovative solutions, the collaboration between manufacturers and medical firms becomes crucial, thereby enhancing the overall efficiency and effectiveness of healthcare delivery.
Regulatory Compliance and Quality Standards
In the US Medical Sector Contract Electronic Manufacturing Market, adherence to stringent regulatory compliance and quality standards is paramount. The Food and Drug Administration (FDA) imposes rigorous guidelines that manufacturers must follow to ensure the safety and efficacy of medical devices. This regulatory landscape compels contract manufacturers to invest in quality management systems and certifications, such as ISO 13485. As a result, companies that prioritize compliance are likely to gain a competitive edge, attracting clients who value reliability and safety. The emphasis on quality not only enhances product integrity but also fosters trust among healthcare providers and patients, ultimately driving market growth.
Increased Investment in Research and Development
Investment in research and development (R&D) is a significant driver of growth in the US Medical Sector Contract Electronic Manufacturing Market. As medical technology evolves, companies are allocating substantial resources to innovate and develop new products. This trend is reflected in the rising number of patents filed in the medical device sector, indicating a robust pipeline of new technologies. Contract manufacturers play a crucial role in this ecosystem by providing the necessary expertise and resources to bring innovative ideas to fruition. By collaborating with R&D teams, these manufacturers can help accelerate the development process, ensuring that new medical devices meet market needs and regulatory requirements.
Technological Advancements in Manufacturing Processes
Technological advancements are reshaping the US Medical Sector Contract Electronic Manufacturing Market. Innovations such as automation, artificial intelligence, and advanced robotics are streamlining manufacturing processes, leading to increased efficiency and reduced production costs. For instance, the integration of smart manufacturing technologies allows for real-time monitoring and predictive maintenance, minimizing downtime. As manufacturers adopt these technologies, they can respond more swiftly to market demands and enhance product quality. This trend is particularly relevant as the medical device sector increasingly requires rapid prototyping and customization, positioning contract manufacturers as vital partners in the development of next-generation medical solutions.