Rising Demand for Vaccines
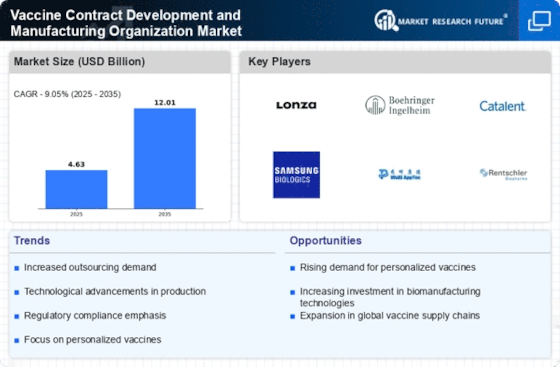
The Vaccine Contract Development and Manufacturing Organization Market is experiencing a notable increase in demand for vaccines, driven by the growing prevalence of infectious diseases and the need for preventive healthcare. According to recent data, the vaccine market is projected to reach approximately 60 billion USD by 2026, indicating a robust growth trajectory. This surge in demand compels organizations to enhance their manufacturing capabilities and expand their production capacities. As a result, vaccine contract development and manufacturing organizations are likely to play a pivotal role in meeting this escalating need, thereby positioning themselves as essential partners for pharmaceutical companies aiming to deliver effective vaccination solutions.
Focus on Personalized Vaccines
The Vaccine Contract Development and Manufacturing Organization Market is witnessing a shift towards personalized vaccines, driven by advancements in genomics and immunology. This trend reflects a growing understanding of the need for tailored vaccination strategies that address individual patient profiles. As research progresses, the demand for contract manufacturers capable of producing personalized vaccines is likely to increase. This shift not only presents new opportunities for innovation but also challenges manufacturers to adapt their processes to accommodate smaller batch sizes and diverse formulations, thereby enhancing their role in the evolving landscape of vaccine development.
Regulatory Support and Funding
The Vaccine Contract Development and Manufacturing Organization Market benefits from increased regulatory support and funding initiatives aimed at bolstering vaccine development. Governments and health organizations are investing significantly in vaccine research and manufacturing infrastructure, which is expected to exceed 10 billion USD in funding over the next few years. This financial backing not only facilitates the development of new vaccines but also enhances the capabilities of contract manufacturers to comply with stringent regulatory standards. Consequently, this trend fosters a conducive environment for innovation and efficiency within the vaccine manufacturing sector, ultimately benefiting public health outcomes.
Strategic Partnerships and Collaborations
Strategic partnerships and collaborations are becoming increasingly prevalent within the Vaccine Contract Development and Manufacturing Organization Market. Pharmaceutical companies are seeking to leverage the expertise and capabilities of contract manufacturers to expedite vaccine development and production. These collaborations often involve sharing resources, knowledge, and technology, which can lead to more efficient processes and faster time-to-market for new vaccines. As the industry continues to evolve, such partnerships are expected to become a cornerstone of vaccine development strategies, enabling organizations to navigate complex regulatory landscapes and meet market demands effectively.
Technological Advancements in Manufacturing
Technological advancements are transforming the Vaccine Contract Development and Manufacturing Organization Market, enabling more efficient and scalable production processes. Innovations such as continuous manufacturing, automation, and advanced bioprocessing techniques are being adopted to enhance productivity and reduce costs. For instance, the implementation of single-use technologies has been shown to decrease production time and minimize contamination risks. As these technologies evolve, they are likely to provide contract manufacturers with a competitive edge, allowing them to meet the increasing demands of vaccine production while maintaining high-quality standards.