Expansion of Healthcare Infrastructure
The expansion of healthcare infrastructure in the United States is a key driver for the transradial access-devices market. As hospitals and clinics invest in modern facilities and advanced medical technologies, the availability of transradial access devices is likely to increase. This expansion is supported by government initiatives aimed at improving healthcare access and quality. With more healthcare facilities equipped to perform transradial procedures, the market is expected to witness substantial growth, reflecting the ongoing commitment to enhancing healthcare delivery across the nation.
Technological Innovations in Medical Devices
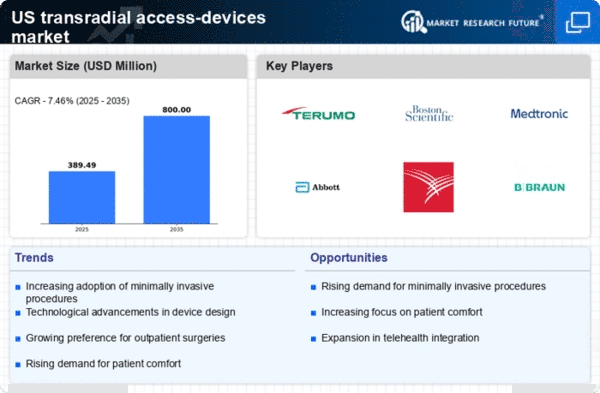
Technological advancements play a crucial role in shaping the transradial access-devices market. Innovations such as advanced imaging techniques, improved catheter designs, and enhanced safety features are driving the adoption of these devices. For instance, the introduction of next-generation catheters with better maneuverability and flexibility has led to increased procedural success rates. The market for these innovative devices is expected to reach $1.5 billion by 2026, indicating a robust growth trajectory. As healthcare providers seek to improve patient outcomes, the demand for technologically advanced transradial access devices is likely to rise.
Growing Prevalence of Cardiovascular Diseases
The transradial access-devices market is significantly influenced by the rising prevalence of cardiovascular diseases in the United States. With heart disease remaining a leading cause of mortality, the demand for effective diagnostic and interventional procedures is increasing. Data suggests that approximately 697,000 deaths occur annually due to heart disease, prompting healthcare systems to adopt more efficient treatment methods. This trend is likely to drive the growth of the transradial access-devices market, as these devices are essential for performing various cardiovascular interventions, thereby addressing the urgent need for effective healthcare solutions.
Increased Focus on Patient Safety and Comfort
Patient safety and comfort are becoming paramount in healthcare, influencing the transradial access-devices market. The shift towards procedures that minimize patient discomfort and reduce the risk of complications is driving the adoption of transradial access techniques. Studies indicate that transradial access is associated with lower rates of bleeding and vascular complications compared to traditional femoral access. As healthcare providers prioritize patient-centered care, the demand for devices that enhance safety and comfort is expected to grow, further propelling the transradial access-devices market.
Rising Demand for Minimally Invasive Procedures
The transradial access-devices market is experiencing a notable increase in demand due to the growing preference for minimally invasive procedures among healthcare providers and patients. This trend is driven by the advantages associated with transradial access, such as reduced recovery time, lower complication rates, and enhanced patient comfort. According to recent data, minimally invasive procedures are projected to grow at a CAGR of approximately 8% over the next five years. As more healthcare facilities adopt these techniques, the transradial access-devices market is likely to expand, reflecting the shift towards less invasive surgical options.