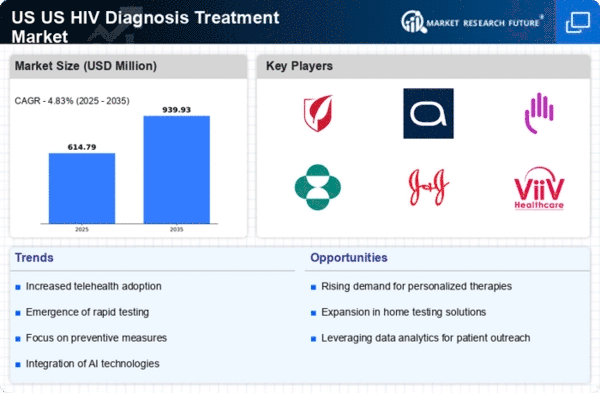
Rising Prevalence of HIV
The US HIV Diagnosis Treatment Market Industry is experiencing growth due to the rising prevalence of HIV infections across the country. According to the Centers for Disease Control and Prevention (CDC), approximately 1.2 million people in the US are living with HIV, with around 14% unaware of their infection status. This alarming statistic underscores the necessity for enhanced diagnostic and treatment options. As awareness increases, more individuals seek testing and treatment, driving demand for innovative solutions. The market is likely to expand as healthcare providers focus on early diagnosis and effective treatment strategies, which are crucial in managing the disease and reducing transmission rates. Furthermore, the increasing number of new diagnoses each year necessitates a robust response from the healthcare system, thereby propelling the US HIV Diagnosis Treatment Market Industry forward.
Growing Awareness and Education
The US HIV Diagnosis Treatment Market Industry is benefiting from a growing awareness and education surrounding HIV/AIDS. Public health campaigns and educational programs have significantly increased knowledge about HIV transmission, prevention, and treatment options. Organizations such as the CDC and various non-profits are actively working to destigmatize HIV testing and treatment, encouraging individuals to seek care. This heightened awareness is likely to lead to increased testing rates, which in turn drives demand for diagnostic and treatment services. Furthermore, educational initiatives targeting high-risk populations are essential in promoting early diagnosis and treatment adherence. As more individuals become informed about their health, the US HIV Diagnosis Treatment Market Industry is expected to see a positive impact, with more people engaging in preventive measures and seeking timely treatment.
Emergence of Personalized Medicine
The emergence of personalized medicine is reshaping the US HIV Diagnosis Treatment Market Industry. Tailoring treatment regimens to individual patient profiles, including genetic factors and drug resistance patterns, enhances the effectiveness of HIV therapies. This approach not only improves patient outcomes but also minimizes adverse effects, leading to higher treatment adherence rates. The increasing availability of pharmacogenomic testing allows healthcare providers to make informed decisions regarding the most suitable treatment options for their patients. As personalized medicine continues to gain traction, it is likely to drive innovation within the market, prompting the development of new therapies and diagnostic tools. The focus on individualized care aligns with broader trends in healthcare, emphasizing the importance of patient-centered approaches in managing chronic diseases like HIV.
Government Initiatives and Funding
Government initiatives play a pivotal role in shaping the US HIV Diagnosis Treatment Market Industry. The federal government, through programs such as the Ryan White HIV/AIDS Program, allocates substantial funding to support HIV care and treatment services. In recent years, there has been a concerted effort to increase funding for HIV prevention and treatment programs, which has led to improved access to diagnostic services. The National HIV/AIDS Strategy aims to reduce new infections and improve health outcomes for those living with HIV, thereby fostering a supportive environment for market growth. Additionally, state-level initiatives often complement federal efforts, creating a comprehensive framework for addressing HIV in the US. This synergy between federal and state programs is likely to enhance the overall effectiveness of the US HIV Diagnosis Treatment Market Industry.
Technological Innovations in Diagnostics
Technological advancements are significantly influencing the US HIV Diagnosis Treatment Market Industry. Innovations such as rapid testing kits, point-of-care testing, and home testing options have revolutionized the way HIV is diagnosed. These technologies not only facilitate quicker results but also enhance accessibility, particularly in underserved communities. The integration of artificial intelligence and machine learning in diagnostic processes is also emerging, potentially improving accuracy and efficiency. As these technologies continue to evolve, they are expected to drive market growth by making testing more convenient and widespread. The increasing adoption of telehealth services further complements these advancements, allowing patients to receive timely diagnoses and treatment recommendations remotely. Consequently, the US HIV Diagnosis Treatment Market Industry is poised for expansion as these innovations become more prevalent.