Rising Demand for Personalized Medicine
The trend towards personalized medicine is reshaping the brugada syndrome market. As genetic testing becomes more accessible, healthcare providers are increasingly able to tailor treatments based on individual patient profiles. This shift is particularly relevant for conditions like Brugada syndrome, where genetic factors play a crucial role in disease manifestation. The market for personalized therapies is projected to grow significantly, with estimates indicating a potential increase of 15% annually. This demand for customized treatment plans is likely to drive the development of targeted therapies and diagnostic tools, enhancing the overall landscape of the brugada syndrome market.
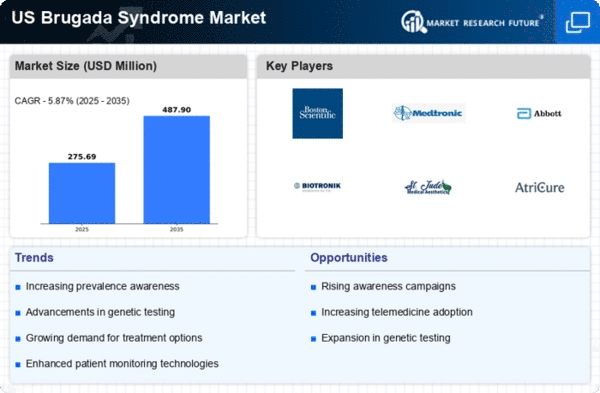
Increasing Incidence of Brugada Syndrome
The rising incidence of Brugada syndrome in the US is a notable driver for the brugada syndrome market. Recent studies indicate that the prevalence of this condition may be higher than previously estimated, with some reports suggesting that it affects approximately 5 in 10,000 individuals. This increase in diagnosed cases is likely to lead to a greater demand for diagnostic tools and treatment options, thereby expanding the market. As healthcare providers become more aware of the syndrome, the number of patients seeking medical attention is expected to rise, further propelling the growth of the brugada syndrome market. Additionally, the need for specialized care and management strategies for affected individuals may create new opportunities for market players, including pharmaceutical companies and medical device manufacturers.
Growing Investment in Research and Development
Investment in research and development (R&D) for Brugada syndrome is a critical driver of the brugada syndrome market. Pharmaceutical companies and research institutions are focusing on understanding the genetic and molecular mechanisms underlying the syndrome. This investment is expected to lead to the development of novel therapies and diagnostic tools. In the US, funding for cardiovascular research has seen a notable increase, with federal and private sources contributing millions of dollars annually. This influx of capital is likely to accelerate the pace of innovation in the brugada syndrome market, ultimately improving patient outcomes and expanding treatment options available to healthcare providers.
Technological Innovations in Cardiac Monitoring
Technological advancements in cardiac monitoring devices are significantly influencing the brugada syndrome market. Innovations such as wearable ECG monitors and implantable loop recorders are enhancing the ability to detect arrhythmias associated with Brugada syndrome. These devices provide continuous monitoring, allowing for timely intervention and management of patients. The market for these technologies is projected to grow, with estimates suggesting a compound annual growth rate (CAGR) of around 8% over the next five years. As healthcare providers increasingly adopt these advanced monitoring solutions, the demand for related services and products in the brugada syndrome market is likely to increase, benefiting both patients and healthcare systems.
Enhanced Awareness Among Healthcare Professionals
Enhanced awareness among healthcare professionals regarding Brugada syndrome is a pivotal driver for the brugada syndrome market. Educational initiatives and training programs are being implemented to improve the recognition and management of this condition. As more healthcare providers become knowledgeable about the syndrome, the likelihood of early diagnosis and appropriate treatment increases. This heightened awareness is expected to lead to a rise in patient referrals and consultations, thereby expanding the market. Furthermore, as guidelines for the management of Brugada syndrome evolve, healthcare professionals will be better equipped to address the needs of affected patients, ultimately contributing to the growth of the brugada syndrome market.