Increased Research Funding
The surge in research funding for rare cardiac conditions, including Brugada syndrome, is a critical driver for the market. In the UK, government and private institutions are allocating more resources to understand the genetic and environmental factors contributing to this syndrome. This influx of funding is expected to facilitate clinical trials and the development of new therapeutic options. As research progresses, it may lead to breakthroughs in treatment protocols, thereby expanding the brugada syndrome market. The focus on personalized medicine, driven by genetic insights, could also enhance the efficacy of interventions, making them more appealing to healthcare providers and patients alike.
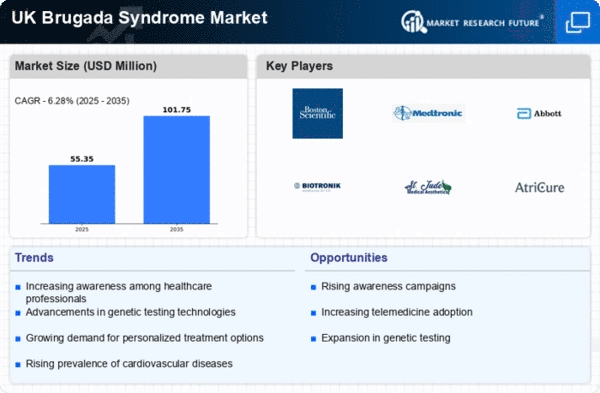
Rising Incidence of Brugada Syndrome
The increasing incidence of Brugada syndrome in the UK is a notable driver for the market. Recent studies indicate that the prevalence of this condition may be higher than previously estimated, with some reports suggesting that it affects approximately 5 in 10,000 individuals. This rise in cases necessitates enhanced diagnostic and treatment options, thereby stimulating growth in the brugada syndrome market. As healthcare providers become more vigilant in identifying this syndrome, the demand for specialized medical devices and therapies is likely to increase. Furthermore, the growing awareness among healthcare professionals about the implications of Brugada syndrome could lead to earlier diagnosis and intervention, which may further drive market expansion.
Growing Patient Advocacy and Support Groups
The emergence of patient advocacy and support groups dedicated to Brugada syndrome is fostering a more informed patient population in the UK. These organizations play a vital role in raising awareness about the condition, promoting early diagnosis, and advocating for better treatment options. As these groups gain traction, they are likely to influence healthcare policies and funding allocations, which could positively impact the brugada syndrome market. Increased patient engagement may also lead to higher demand for specialized care and innovative therapies, as individuals become more proactive in managing their health. This shift in patient dynamics is expected to create new opportunities for market players.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies targeting rare cardiac conditions is emerging as a significant driver in the brugada syndrome market. The UK regulatory framework is increasingly favorable towards expedited approval processes for novel treatments, particularly those addressing unmet medical needs. This supportive environment encourages pharmaceutical companies to invest in research and development for Brugada syndrome therapies. As a result, the market may witness a rise in the availability of effective treatment options, which could enhance patient outcomes. The potential for faster market entry of new drugs and devices is likely to attract investment and stimulate growth within the brugada syndrome market.
Technological Advancements in Cardiac Monitoring
Technological advancements in cardiac monitoring devices are significantly influencing the brugada syndrome market. Innovations such as wearable ECG monitors and implantable loop recorders are becoming increasingly prevalent in the UK. These devices enable continuous monitoring of patients at risk of arrhythmias associated with Brugada syndrome. The market for these technologies is projected to grow, with estimates suggesting a compound annual growth rate (CAGR) of around 8% over the next five years. As these technologies become more accessible and affordable, healthcare providers are likely to adopt them more widely, enhancing patient management and potentially reducing the incidence of life-threatening events.