Increasing Incidence of Brugada Syndrome
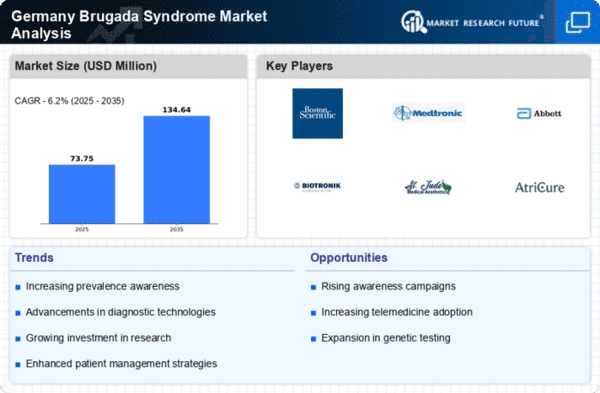
The rising incidence of Brugada syndrome in Germany is a crucial driver for the Brugada syndrome market. Recent studies indicate that the prevalence of this condition may be higher than previously estimated, with some reports suggesting that it affects approximately 5 in 10,000 individuals. This increase in diagnosed cases is likely to stimulate demand for diagnostic tools and treatment options, thereby expanding the market. Furthermore, as awareness grows among healthcare professionals and patients, more individuals are likely to seek medical advice, leading to earlier diagnosis and intervention. This trend could potentially enhance the overall market landscape, as healthcare providers may invest in advanced technologies and therapies to address the needs of this patient population.
Increased Collaboration Among Stakeholders
Increased collaboration among stakeholders in the healthcare ecosystem is emerging as a significant driver for the brugada syndrome market. Partnerships between academic institutions, healthcare providers, and industry players are fostering a multidisciplinary approach to research and treatment. In Germany, collaborative initiatives are being established to share knowledge, resources, and expertise, which can lead to more effective management strategies for Brugada syndrome. These collaborations may result in joint clinical trials, shared databases, and the development of standardized treatment protocols. As stakeholders work together to address the complexities of this condition, the market is likely to benefit from enhanced innovation and improved patient outcomes, ultimately driving growth in the brugada syndrome market.
Regulatory Framework Supporting Market Growth
The regulatory framework in Germany plays a pivotal role in shaping the Brugada syndrome market. The European Medicines Agency (EMA) and the Federal Institute for Drugs and Medical Devices (BfArM) provide guidelines that facilitate the approval of new therapies and diagnostic tools. This supportive environment encourages pharmaceutical companies and medical device manufacturers to invest in the development of innovative solutions for Brugada syndrome. Moreover, streamlined approval processes can lead to faster market entry for new products, which is essential for addressing the urgent needs of patients. As regulatory bodies continue to prioritize patient safety and efficacy, the brugada syndrome market is likely to experience sustained growth, driven by the introduction of novel therapies and technologies.
Growing Investment in Research and Development
The growing investment in research and development (R&D) within the brugada syndrome market is a notable driver. In Germany, public and private sectors are increasingly funding studies aimed at understanding the genetic and environmental factors contributing to Brugada syndrome. This influx of capital is likely to accelerate the development of novel therapies and diagnostic tools. For instance, recent funding initiatives have allocated millions of euros to research projects focused on identifying biomarkers and potential treatment pathways. As new findings emerge, they may lead to innovative solutions that address unmet medical needs, thereby expanding the market. The commitment to R&D not only enhances the scientific understanding of the condition but also positions Germany as a leader in the development of cutting-edge therapies.
Technological Advancements in Cardiac Monitoring
Technological advancements in cardiac monitoring devices are significantly influencing the brugada syndrome market. Innovations such as wearable ECG monitors and implantable loop recorders are becoming increasingly prevalent in Germany. These devices allow for continuous monitoring of cardiac rhythms, which is essential for detecting arrhythmias associated with Brugada syndrome. The integration of telemedicine and remote monitoring solutions further enhances patient management, enabling timely interventions. As the demand for these advanced monitoring solutions grows, manufacturers are likely to invest in research and development, leading to a broader range of products available in the market. This evolution in technology not only improves patient outcomes but also drives market growth by attracting investments and fostering competition among key players.