Regulatory Support and Guidelines
The US Actinic Keratosis Treatment Market benefits from supportive regulatory frameworks and guidelines that facilitate the approval and adoption of new treatments. The Food and Drug Administration (FDA) has established clear pathways for the approval of novel therapies, which encourages pharmaceutical companies to invest in research and development. Additionally, the National Comprehensive Cancer Network (NCCN) provides guidelines that recommend specific treatment options for actinic keratosis, further standardizing care and promoting the use of effective therapies. This regulatory support not only enhances patient access to innovative treatments but also fosters a competitive market environment, driving growth in the industry.
Integration of Telehealth Services
The US Actinic Keratosis Treatment Market is increasingly benefiting from the integration of telehealth services, which enhance access to care for patients. Telemedicine allows dermatologists to conduct virtual consultations, making it easier for patients to receive timely evaluations and treatment recommendations without the need for in-person visits. This is particularly advantageous for individuals living in rural or underserved areas, where access to dermatological care may be limited. The convenience and efficiency of telehealth services are likely to encourage more patients to seek treatment for actinic keratosis, thereby expanding the market. As telehealth continues to evolve, it may play a crucial role in shaping the future landscape of the US Actinic Keratosis Treatment Market.
Advancements in Treatment Modalities
The US Actinic Keratosis Treatment Market is witnessing a surge in innovative treatment modalities, which enhances patient outcomes and satisfaction. Recent advancements include the development of topical therapies, photodynamic therapy, and laser treatments, which have shown promising results in clinical trials. For instance, the introduction of new topical agents, such as ingenol mebutate and 5-fluorouracil, has provided patients with effective options that are less invasive than traditional surgical methods. The market is also seeing an increase in combination therapies, which may improve efficacy and reduce recurrence rates. As these treatment options become more widely available, they are likely to attract more patients seeking effective solutions for actinic keratosis.
Growing Focus on Skin Cancer Prevention
The US Actinic Keratosis Treatment Market is significantly influenced by the growing emphasis on skin cancer prevention. Public health campaigns and educational initiatives aimed at raising awareness about the risks associated with UV exposure have led to increased screening and early intervention for actinic keratosis. The Centers for Disease Control and Prevention (CDC) has reported that skin cancer is the most common cancer in the United States, which has prompted healthcare providers to prioritize the treatment of actinic keratosis as a preventive measure. This proactive approach not only helps in reducing the incidence of skin cancer but also drives the demand for effective treatments within the market.
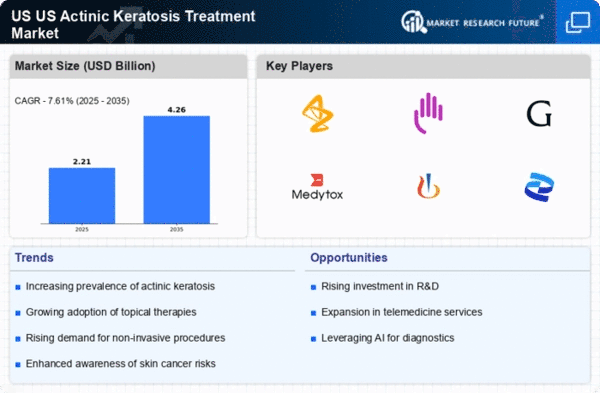
Increasing Incidence of Actinic Keratosis
The US Actinic Keratosis Treatment Market is experiencing growth due to the rising incidence of actinic keratosis, particularly among older adults. According to the American Academy of Dermatology, approximately 58 million Americans are affected by actinic keratosis, which is often considered a precursor to skin cancer. This increasing prevalence necessitates effective treatment options, driving demand within the market. Furthermore, the aging population in the United States is projected to grow significantly, with estimates suggesting that by 2030, one in five Americans will be over the age of 65. This demographic shift is likely to contribute to a higher incidence of actinic keratosis, thereby expanding the market for treatments aimed at this condition.
.png)