Aging Population
The aging population in the UK is a significant driver of the precision medical devices market. As the demographic shifts towards an older population, there is an increasing prevalence of chronic diseases and age-related conditions that require advanced medical interventions. According to the Office for National Statistics, the proportion of individuals aged 65 and over is projected to rise to 23% by 2030. This demographic trend is likely to increase the demand for precision medical devices that cater to the unique healthcare needs of older adults. Consequently, manufacturers are focusing on developing devices that enhance the quality of life for this population, such as home monitoring systems and assistive technologies, thereby driving growth in the market.
Regulatory Evolution
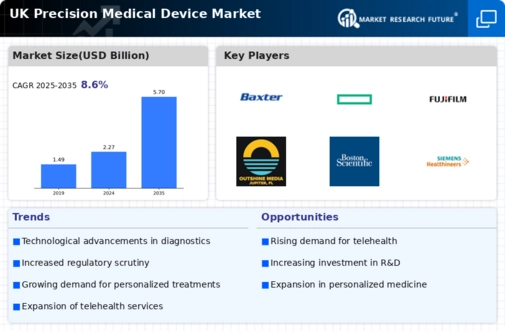
The regulatory landscape for the UK precision medical devices market is evolving, with the UK Medicines and Healthcare products Regulatory Agency (MHRA) implementing new guidelines to ensure safety and efficacy. These regulations are designed to streamline the approval process for innovative devices while maintaining high safety standards. The introduction of the UKCA mark, which replaces the CE mark post-Brexit, has created a need for manufacturers to adapt to new compliance requirements. This regulatory evolution may initially pose challenges, but it also presents opportunities for companies to differentiate themselves through compliance and innovation. As the market adapts, it is expected that the regulatory framework will foster a more competitive environment, encouraging the development of cutting-edge precision medical devices.
Technological Advancements
The UK precision medical devices market is experiencing rapid technological advancements that are reshaping healthcare delivery. Innovations such as artificial intelligence, machine learning, and advanced imaging techniques are enhancing diagnostic accuracy and treatment efficacy. For instance, the integration of AI in imaging devices has led to a 30% increase in diagnostic precision, significantly impacting patient outcomes. Furthermore, the development of minimally invasive surgical tools is reducing recovery times and hospital stays, which is particularly beneficial in the UK healthcare system. As these technologies continue to evolve, they are likely to drive growth in the precision medical devices sector, attracting investments and fostering collaborations between tech companies and healthcare providers.
Focus on Personalized Medicine
The increasing emphasis on personalized medicine is a key driver in the UK precision medical devices market. Tailored treatment approaches, which consider individual patient characteristics, are becoming more prevalent, leading to the development of devices that cater to specific patient needs. For example, advancements in genomics and biomarker identification are enabling the creation of precision therapies that improve treatment outcomes. The UK government has recognized the importance of personalized medicine, investing in initiatives that support research and development in this area. This focus not only enhances patient care but also stimulates growth in the precision medical devices sector, as companies strive to innovate and meet the demands of a more personalized healthcare landscape.
Investment in Healthcare Infrastructure
Investment in healthcare infrastructure is a crucial driver for the UK precision medical devices market. The UK government has committed to increasing funding for healthcare services, which includes the procurement of advanced medical technologies. The NHS Long Term Plan outlines significant investments aimed at modernizing facilities and integrating innovative medical devices into patient care. This commitment to enhancing healthcare infrastructure is expected to create a favorable environment for the adoption of precision medical devices. As hospitals and clinics upgrade their equipment and technology, the demand for state-of-the-art precision devices is likely to rise, fostering growth and innovation within the industry.