Rising Public Awareness
Rising public awareness regarding drug safety and adverse effects is a crucial driver for the pharmacovigilance market in the UK. As patients become more informed about their medications, there is an increasing expectation for pharmaceutical companies to provide transparent information about potential risks. This shift in consumer behavior is prompting companies to enhance their pharmacovigilance efforts, ensuring that they can respond effectively to public concerns. The market is likely to see a growth rate of around 6% as organizations prioritize communication and education regarding drug safety. This heightened awareness not only influences corporate strategies but also shapes regulatory policies, ultimately leading to improved pharmacovigilance practices.
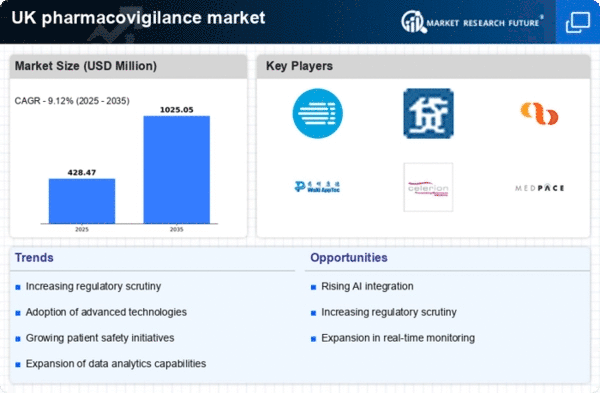
Increasing Regulatory Scrutiny
The pharmacovigilance market in the UK is experiencing heightened regulatory scrutiny, which is driving the demand for robust safety monitoring systems. Regulatory bodies, such as the Medicines and Healthcare products Regulatory Agency (MHRA), are enforcing stricter guidelines for drug safety reporting. This has led to an increase in the adoption of advanced pharmacovigilance solutions to ensure compliance. The market is projected to grow at a CAGR of approximately 8% over the next few years, as companies invest in technologies that facilitate real-time monitoring and reporting of adverse drug reactions. The emphasis on regulatory compliance is likely to enhance the overall safety of pharmaceuticals, thereby fostering trust among healthcare professionals and patients alike.
Expansion of Biopharmaceuticals
The expansion of biopharmaceuticals is significantly impacting the pharmacovigilance market in the UK. As the biopharmaceutical sector continues to grow, the complexity of monitoring the safety of these products increases. Biologics often have unique safety profiles, necessitating specialized pharmacovigilance strategies. The UK market is witnessing a surge in the development of biopharmaceuticals, which is projected to account for over 30% of the total pharmaceutical market by 2026. This growth is driving the need for enhanced pharmacovigilance practices to ensure the safety and efficacy of these innovative therapies. Consequently, companies are investing in advanced monitoring systems to manage the unique challenges posed by biopharmaceuticals.
Growing Demand for Patient Safety
The growing emphasis on patient safety is a significant driver for the pharmacovigilance market in the UK. With an increasing number of patients becoming aware of their rights and the importance of drug safety, there is a rising demand for transparent reporting of adverse drug reactions. This trend is prompting pharmaceutical companies to invest in comprehensive pharmacovigilance systems that prioritize patient safety. According to recent estimates, the market is expected to reach approximately £1 billion by 2027, reflecting a compound annual growth rate of 7%. The focus on patient-centric approaches in pharmacovigilance not only enhances drug safety but also improves patient trust in healthcare systems.
Integration of Artificial Intelligence
The integration of artificial intelligence (AI) in the pharmacovigilance market is transforming how adverse events are monitored and reported. AI technologies enable the analysis of vast datasets, identifying patterns and potential safety signals more efficiently than traditional methods. In the UK, the adoption of AI-driven solutions is expected to increase, with market analysts estimating a growth rate of around 10% annually. This technological advancement not only streamlines the pharmacovigilance processes but also enhances the accuracy of data interpretation. As pharmaceutical companies seek to improve their operational efficiency and reduce costs, the incorporation of AI into pharmacovigilance practices is likely to become a standard approach.