Regulatory Compliance and Safety Standards
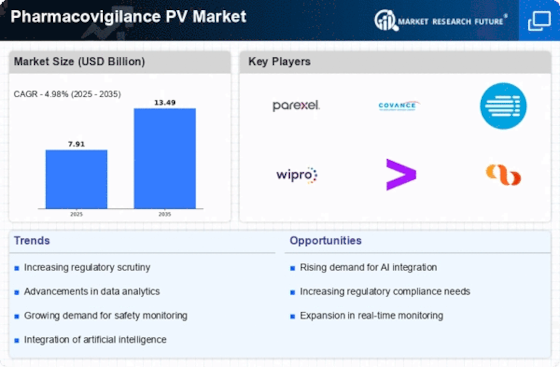
The increasing emphasis on regulatory compliance and safety standards is a pivotal driver for the Pharmacovigilance PV Market. Regulatory bodies, such as the FDA and EMA, have established stringent guidelines to ensure drug safety and efficacy. This has led to a heightened demand for robust pharmacovigilance systems that can efficiently monitor adverse drug reactions and ensure compliance with these regulations. As of 2025, the market for pharmacovigilance services is projected to reach approximately USD 8 billion, reflecting a compound annual growth rate of around 10%. Companies are investing in advanced technologies to streamline reporting processes and enhance data accuracy, thereby reinforcing their commitment to patient safety and regulatory adherence.
Rising Incidence of Adverse Drug Reactions
The rising incidence of adverse drug reactions (ADRs) is a significant driver for the Pharmacovigilance PV Market. With the increasing complexity of drug formulations and the growing number of medications available, the likelihood of ADRs has escalated. Reports indicate that ADRs account for a substantial percentage of hospital admissions, prompting healthcare providers and pharmaceutical companies to prioritize pharmacovigilance efforts. In 2025, the market is expected to witness a surge in demand for comprehensive monitoring systems that can effectively track and analyze ADRs. This trend underscores the necessity for enhanced pharmacovigilance practices to mitigate risks associated with drug therapies and ensure patient safety.
Increased Focus on Patient-Centric Approaches
The increased focus on patient-centric approaches is a notable driver for the Pharmacovigilance PV Market. As healthcare evolves, there is a growing recognition of the importance of patient feedback in the pharmacovigilance process. Engaging patients in reporting adverse events and understanding their experiences with medications is becoming essential for improving drug safety. This trend is likely to enhance the quality of safety data collected, leading to more effective risk management strategies. By 2025, the emphasis on patient-centric pharmacovigilance is expected to contribute to market growth, with projections indicating a market size of approximately USD 8.5 billion. This shift reflects a broader commitment to prioritizing patient safety and satisfaction in the healthcare landscape.
Technological Advancements in Data Management
Technological advancements in data management are transforming the Pharmacovigilance PV Market. The integration of artificial intelligence, machine learning, and big data analytics is enabling organizations to process vast amounts of safety data more efficiently. These technologies facilitate real-time monitoring of drug safety profiles, allowing for quicker identification of potential risks. As of October 2025, the adoption of these advanced technologies is anticipated to drive market growth, with estimates suggesting a market value exceeding USD 9 billion. The ability to harness data effectively not only enhances the pharmacovigilance processes but also supports regulatory compliance and improves overall patient outcomes.
Growing Demand for Outsourced Pharmacovigilance Services
The growing demand for outsourced pharmacovigilance services is reshaping the Pharmacovigilance PV Market. Pharmaceutical companies are increasingly recognizing the benefits of outsourcing these services to specialized firms that possess the expertise and resources to manage safety data effectively. This trend is driven by the need for cost efficiency, access to advanced technologies, and the ability to focus on core business activities. By 2025, the market for outsourced pharmacovigilance services is projected to grow significantly, with estimates indicating a value of around USD 7 billion. This shift allows companies to enhance their pharmacovigilance capabilities while ensuring compliance with regulatory requirements.