Focus on Patient-Centric Approaches
The shift towards patient-centric approaches in healthcare is significantly influencing the Pharmacovigilance and Drug Safety Software Market. As stakeholders prioritize patient safety and experience, there is a growing need for software that can effectively capture and analyze patient-reported outcomes. This trend is likely to drive the development of more intuitive and user-friendly software solutions. Companies that can leverage patient data to enhance drug safety monitoring are expected to gain a competitive edge. The increasing emphasis on personalized medicine also suggests that pharmacovigilance solutions will need to adapt to diverse patient populations, further expanding the market.
Integration of Advanced Technologies
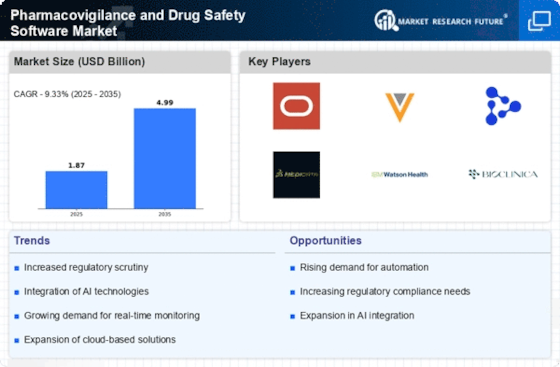
The integration of advanced technologies such as artificial intelligence and machine learning is transforming the Pharmacovigilance and Drug Safety Software Market. These technologies enhance data analysis capabilities, enabling quicker identification of adverse drug reactions and improving overall safety monitoring. As organizations increasingly adopt these technologies, the market is projected to grow significantly. For instance, the market for AI in drug safety is expected to reach substantial figures by 2026, indicating a robust demand for innovative solutions. This trend not only streamlines processes but also reduces the time required for regulatory submissions, thereby enhancing compliance and operational efficiency.
Regulatory Compliance and Standardization
Regulatory compliance remains a critical driver in the Pharmacovigilance and Drug Safety Software Market. With stringent regulations imposed by health authorities, pharmaceutical companies are compelled to adopt software solutions that ensure adherence to safety standards. The increasing complexity of regulatory requirements necessitates sophisticated software that can manage vast amounts of data while ensuring compliance. The market is likely to witness growth as companies invest in solutions that facilitate real-time reporting and monitoring of drug safety. Furthermore, the harmonization of regulations across regions may lead to a more streamlined approach, further driving the demand for effective pharmacovigilance solutions.
Rising Incidence of Adverse Drug Reactions
The rising incidence of adverse drug reactions (ADRs) is a significant driver for the Pharmacovigilance and Drug Safety Software Market. As the number of medications in use continues to grow, so does the potential for ADRs, prompting healthcare providers and regulatory bodies to prioritize drug safety. This trend is likely to lead to increased investments in pharmacovigilance software that can efficiently track and analyze ADR data. The market is projected to expand as organizations seek to mitigate risks associated with drug therapies. Enhanced reporting capabilities and data analytics are essential for addressing this challenge, thereby driving demand for advanced software solutions.
Growing Demand for Real-Time Data Analytics
The growing demand for real-time data analytics is reshaping the Pharmacovigilance and Drug Safety Software Market. Stakeholders are increasingly recognizing the value of timely insights into drug safety, which can significantly impact patient outcomes. As a result, there is a heightened interest in software solutions that offer real-time monitoring and reporting capabilities. This trend is expected to drive market growth as organizations seek to enhance their pharmacovigilance efforts. The ability to analyze data in real-time allows for quicker decision-making and more effective risk management, which is crucial in today's fast-paced healthcare environment.