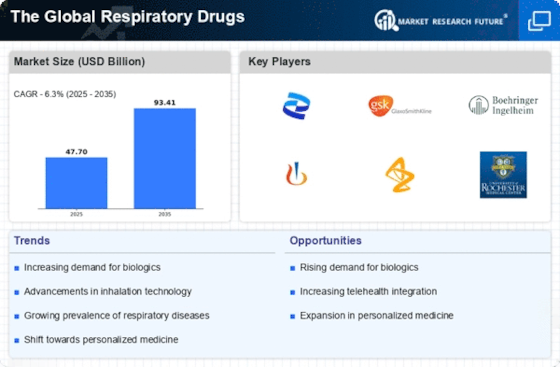
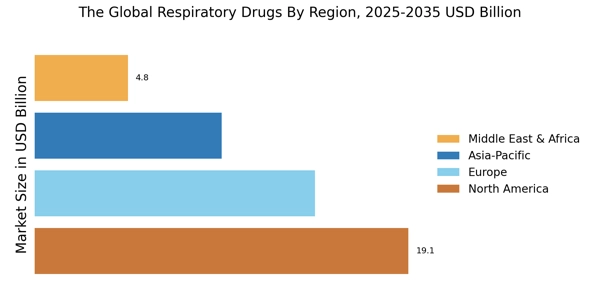
Leading market players are investing heavily in research and development in order to expand their Product lines, which will help the market of Respiratory Drugs Market, grow even more. Market participants are also undertaking a variety of strategic activities to expand their global footprint, with important market developments including new product launches, contractual agreements, mergers and acquisitions, higher investments, and collaboration with other organizations. To expand and survive in a more competitive and rising market climate, Respiratory Drugs Industry must offer cost-effective items. Manufacturing locally to minimize operational costs is one of the key business tactics used by manufacturers in the global Respiratory Drugs Industry to benefit clients and increase the market sector. In recent years, the Respiratory Drugs Industry has offered some of the most significant advantages to medicine. Major players in the Respiratory Drugs Market, including VAPOTHERM, AptarGroup, Inc., Drägerwerk AG & CO., Allied Healthcare, Cardinal Health, NSPIRE HEALTH INC. oninklijke Philips N.V., and others, are attempting to increase market demand by investing in research and development operations. A medical device firm called Niox Group Plc (Niox Group), formerly known as Circassia Group Plc (Circassia), creates and produces medications for the treatment of respiratory conditions like asthma. The company markets medications including Niox, which is used to treat asthma. Through its network of partners, the company provides its products to asthma specialists in the US, the UK, Sweden, Germany, China, and other nations. The Niox Group is based in Oxford, England, in the United Kingdom. Circassia in March 2019. Obstructive pulmonary disease (COPD) maintenance and treatment with Tudoorza has been given FDA approval. Theravance Biopharma Inc. (Theravance Biopharma) is a biopharmaceutical business that specializes in creating respiratory medications and discovers, creates, and markets organ-selective medications for the treatment of immune system and inflammatory illnesses. Asthma, neurogenic orthostatic hypotension (nOH), gastrointestinal motility disorders, ulcerative colitis, heart failure, chronic kidney disease (CKD), gastroparesis, and concurrent bacteremia are among the conditions the company is looking at with its pipeline candidates. Theravance Biopharma has contracts with other pharmaceutical firms and owns financial stakes in prospective future payments from Glaxo Group Limited or one of its affiliates to Innoviva, Inc. The US, Ireland, and the UK all have subsidiaries for the business. San Francisco, California, in the United States, serves as the headquarters for Theravance Biopharma. A new drug application (NDA) for YUPELRITM (revefenacin) inhalation solution for the maintenance treatment of people with chronic obstructive pulmonary disease (COPD) was approved by the FDA in November 2018 by Theravance Biopharma and Mylan N.V.