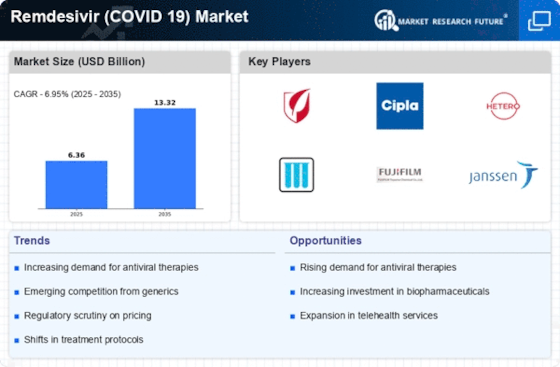
Top Industry Leaders in the Remdesivir Market

Latest Remdesivir Companies Update
July 2023: Remdesivir is the first antiviral medication that the FDA has licensed for the therapy of COVID-19 among individuals with serious kidney damage, including those on dialysis. Randomized controlled studies, real-world proof, and meta-analyses all indicate the clinical value of COVID-19 in hospitalized patients; nevertheless, due to a lack of data, its usage has historically been restricted in patients with serious kidney disease. The revised prescribing information for patients with renal impairment eliminates the need for estimated glomerular filtration rate (eGFR) testing before and throughout remdesivir treatment. Due to concerns with feasibility, the trial closed earlier than planned, and because enrollment was fewer than anticipated, there was insufficient power to evaluate its efficacy.
Aug 2023: Gilead Sciences announced that it has partnered with Tentarix Biotherapeutics, a privately held company, to research treatments for inflammatory and cancerous conditions. Gilead has agreed to make upfront payments to Tentarix and an equity investment of $66 million. Gilead can purchase up to three of the drug developer's units for $80 million. Through the agreement, Gilead will access Tentarix's exclusive drug development platform for antibody-based treatments that selectively target immune cells associated with the disease without activating other immune cells that could cause unfavorable outcomes.List of Remdesivir Key companies in the market
-
Gilead Sciences, Inc. (US)
-
Mylan (US)
-
Cipla (India)
-
Hetero Labs (India)
-
Jubilant Life Sciences (India)