Demand for Enhanced Data Analytics
The demand for enhanced data analytics capabilities is reshaping the Regulatory Information Management Market. Organizations are recognizing the value of data-driven insights in managing regulatory compliance and risk. Advanced analytics tools enable companies to analyze vast amounts of regulatory data, identify trends, and make informed decisions. This trend is particularly relevant in industries such as life sciences, where data analytics can significantly improve regulatory submissions and reporting. As organizations strive for operational efficiency and compliance accuracy, the market for regulatory information management solutions is anticipated to expand, with a focus on integrating advanced analytics.
Focus on Risk Management and Mitigation
The focus on risk management and mitigation is a pivotal driver in the Regulatory Information Management Market. Organizations are increasingly aware of the potential risks associated with non-compliance, which can lead to severe financial penalties and reputational damage. As a result, there is a growing emphasis on implementing comprehensive regulatory information management systems that facilitate proactive risk assessment and management. This trend is particularly evident in sectors such as energy and telecommunications, where regulatory scrutiny is high. The market is expected to grow as organizations prioritize risk management strategies to safeguard their operations and ensure compliance.
Rising Complexity of Regulatory Frameworks
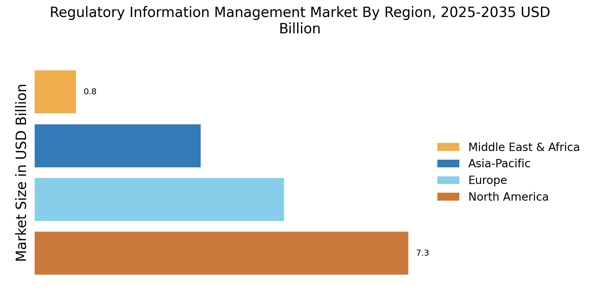
The complexity of regulatory frameworks is a significant driver for the Regulatory Information Management Market. As regulations evolve and become more intricate, organizations face challenges in maintaining compliance. This complexity is particularly pronounced in sectors such as finance and environmental management, where multiple regulatory bodies impose varying requirements. Consequently, companies are increasingly investing in regulatory information management solutions to streamline compliance processes and ensure adherence to diverse regulations. The market is expected to witness substantial growth as organizations seek to navigate this regulatory labyrinth effectively.
Increasing Regulatory Compliance Requirements
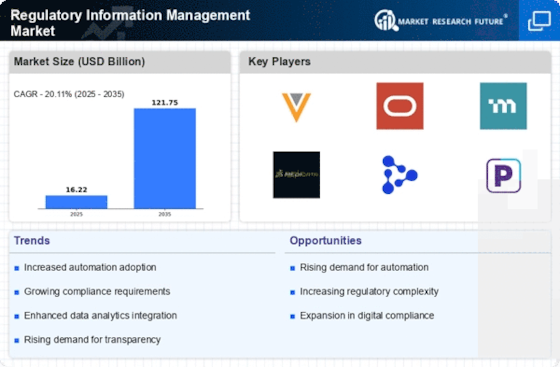
The Regulatory Information Management Market is experiencing a surge in demand due to the increasing regulatory compliance requirements across various sectors. Organizations are compelled to adhere to stringent regulations, which necessitates the implementation of robust regulatory information management systems. This trend is particularly evident in industries such as pharmaceuticals and healthcare, where compliance with regulations like the FDA and EMA is critical. As a result, the market is projected to grow at a compound annual growth rate (CAGR) of approximately 12% over the next five years, driven by the need for organizations to mitigate risks associated with non-compliance.
Adoption of Artificial Intelligence and Automation
The adoption of artificial intelligence (AI) and automation technologies is transforming the Regulatory Information Management Market. Organizations are increasingly leveraging AI to automate compliance processes, enhance data accuracy, and reduce manual errors. This shift is particularly beneficial in industries such as manufacturing and pharmaceuticals, where regulatory requirements are stringent and time-sensitive. By implementing AI-driven solutions, companies can streamline their regulatory workflows, leading to improved efficiency and reduced operational costs. The market is likely to see accelerated growth as more organizations recognize the potential of AI and automation in regulatory information management.