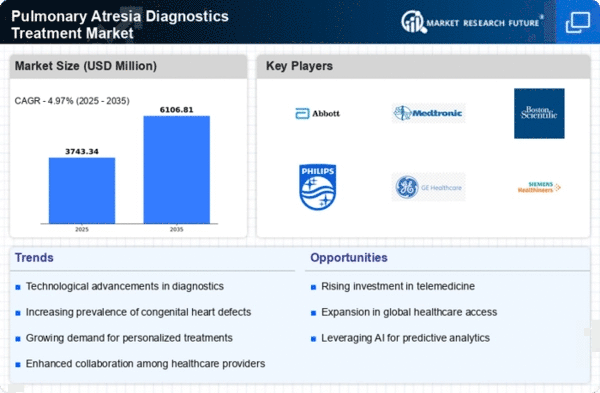
Market Analysis
In-depth Analysis of Pulmonary Atresia Diagnostics Treatment Market Industry Landscape
Pulmonary atresia is a congenital heart disorder characterized by the absence or closure of the pulmonary valve, affecting blood drift from the heart to the lungs. Analyzing the market dynamics of Pulmonary Atresia diagnostics and treatment entails exploring factors including prevalence, diagnostic improvements, treatment modalities, and the aggressive panorama in the healthcare quarter. Understanding market dynamics begins with an exam of the prevalence of pulmonary atresia, particularly its occurrence within the pediatric population. This congenital coronary heart disorder calls for specialized diagnostics and remedies, making it a large consideration for healthcare carriers and pharmaceutical corporations. Market dynamics are motivated by continuous advancements in diagnostic technologies for pulmonary atresia. Improved imaging techniques, together with echocardiography and cardiac MRI, play an important role in correct and early analysis. The market responds to improvements that decorate the precision and efficiency of diagnostic methods. Pharmaceutical agencies contribute to market dynamics through improvements in medications for pulmonary atresia. Drugs that improve cardiac features, control signs, and decorate standard coronary heart health are essential components of the remedy panorama. Research and improvement efforts recognize the unique, demanding situations posed by way of pulmonary atresia. The market for Pulmonary Atresia diagnostics and treatment is aggressive, with healthcare providers and pharmaceutical businesses vying for market share. Competition fosters studies and improvement, mainly in the creation of recent diagnostic tools, remedy alternatives, and healing tactics. Companies intend to establish their merchandise as trendy-of-care in the scientific network. Market dynamics are motivated by the specialization of healthcare experts in pediatric cardiology. The knowledge of pediatric cardiologists in handling congenital heart defects, inclusive of pulmonary atresia, is essential in shaping treatment protocols and influencing the adoption of specific interventions. Regulatory approvals and compliance with healthcare standards drastically affect the market dynamics of Pulmonary Atresia diagnostics and treatment. Manufacturers and healthcare companies must navigate regulatory processes to ensure the protection and efficacy of diagnostic gear and treatment modalities. The market is inspired by patient advocacy companies and help groups that play a critical role in raising awareness, imparting assets, and advocating for progressed care for individuals with pulmonary atresia. The state of global healthcare infrastructure plays a role in market dynamics, impacting the right of entry to specialized care for pulmonary atresia patients. The integration of the era in healthcare, along with telemedicine and faraway monitoring, is a growing trend that influences the market dynamics for pulmonary atresia. Remote consultations, digital fitness statistics, and telemedicine solutions contribute to progressed accessibility and continuity of care.

















Leave a Comment